Some Aspects of Role of Nitric Oxide in the Mechanisms of Hypertension (Experimental Study)
Abstract
Background
Modulation of endothelial function is a therapeutic option to reduce some of the significant complications of hypertension. However, the relationship between endothelial dysfunction reduced nitric oxide (NO) production, and the development of hypertension is not fully understood. To establish a potential pathogenetic link between impaired NO synthesis and hypertension, we investigated the results of competitive interaction of the substrate of NO synthase, L-arginine, and its analog, an non-selective inhibitor of NO synthase, N-nitro-methyl ether-L-arginine (L-NAME), in experimental rats.
Methods
Arterial hypertension was induced in male Wistar rats by intraperitoneal administration of L-NAME (Sigma-Aldrich) for 4 – 7 weeks. During the last 3 weeks, to a separate group of animals simultaneously with L-NAME, L-arginine (Sigma-Aldrich) was administered. In animals monitored for systolic and diastolic pressure, the level of NO in blood samples was determined spectrophotometrically using a Griess reagent.
Results
Administration of L-NAME for 4 – 7 weeks induced an irreversible decrease of NO content in blood, a reversible increase of systolic pressure (SP) and diastolic pressure (DP), and an irreversible increase in pulse pressure (PP). In rats against the background of 7 weeks of intraperitoneal administration of L-NAME, during the last 3 weeks, they were injected with L-arginine, the SP and DP indices returned to their initial values, PP decreased and the NO content in arterial blood increased.
Conclusions
The results of the study indicate the presence of residual endothelial dysfunction (characterized by insufficient NO) after the correction of hypertension. Therefore, in developing the new therapeutic approaches for the treatment of hypertension, it is necessary to include drugs that, in addition to correcting blood pressure, will support normalization, and potentiation of endothelial function and endogenous NO synthesis.
Introduction
The etiology of hypertension and its relationship with disorders of nitric oxide (NO) production, the question of whether dysfunction of the endothelium and a decrease in NO production occurs before or after the development of hypertension, remains unclear. Since endothelial dysfunction is usually observed in various types of hypertension and can be restored by correcting blood pressure, it is believed that the extrusion of endothelial function is not a primary cause, but a secondary result of hypertension. However, impaired NO-dependent vasodilation has been shown to precede hypertension in black patients with normotension and in normotensive offsprings of hypertensive parents. Although, due to the modest percentage of the heritability of endothelial dysfunction, measured as flow-mediated dilation of the brachial artery, generally, it is difficult to assert that endothelial dysfunction is a cause of hypertension.
The theory of “endothelial dysfunction”, and consequential insufficient NO production in human with essential hypertension led to the creation of an animal model of human hypertension where NO deficiency, achieved by both acute and chronic inhibition of NO synthase (NOS) with arginine derivatives, induced a significant rise in blood pressure (so-called “NO-deficient hypertension” in normotensive rats), and this model became widely used for investigation of the NO participation in cardiovascular disorders [5].
The mechanism of NOS inhibition by substrate analogs consists of competitive binding to the enzyme. Reversible inhibitor and one of the most frequently used L-arginine substituents is N-nitro-methyl ether-L-arginine (L-NAME), which is considered to be a non-selective inhibitor of NOS (a competitive inhibitor of all NOSs having higher selectivity to endothelial NOS (eNOS) and neuronal NOS (nNOS) over inducible NOS (iNOS). Administration of L-NAME is also associated with increased production of reactive oxygen species (ROS) accompanied by depletion of endogenous antioxidants. Under conditions of intensified intracellular ROS production, oxidation of the tetrahydrobiopterin and other cofactors needed for NO synthesis leads to the uncoupling of the NOS dimer which results in decreased NOS activity. Additionally, in the condition of oxidative stress, NOS produces rather superoxide radical than NO, and oxidative degradation of NO to peroxynitrite is also possible. Therefore, chronic administration of L-NAME in experimental rats is accompanied by a significant decrease in the synthesis of NO.
Modulation of endothelial function is a therapeutic option to reduce some of the significant complications of hypertension. However, the relationship between endothelial dysfunction reduced NO production, and the development of hypertension is not fully understood.
To establish physiological and pathophysiological aspects of NO role in the mechanisms of hypertension, a potential pathogenetic link between impaired NO synthesis and endothelial dysfunction, we investigated the competitive interaction of the substrate of NOS, L-arginine and its analog L-NAME in experimental rats.
Materials and Methods
Animal models of hypertension
In the study, 13- to 14-month-old (body weight 200 – 220 g) white Wistar male rats (35 animals) were used. All animals were kept in acrylic cages with wood shavings in an acclimatized room (12/12 h light/dark cycle; 22 ± 3 °C) with free access to food and water. All animal procedures were approved by the Animal Care and Use Committee of the Tbilisi State Medical University and were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, revised 1996). All efforts were made to minimize the number of animals and their suffering throughout the experiment.
Hypertension was induced with intraperitoneal administration of L-NAME (Sigma-Aldrich) (40 mg/kg).
The animals were divided into three groups. 1) Group I, control group (seven intact rats). 2) Group II, experimental group (an experimental model of hypertension (21 rats) contained three (IIa, IIb and IIc) subgroups (seven rats in each subgroup)): IIa subgroup – intraperitoneal administration of L-NAME (40 mg/kg) during 4 weeks; IIb subgroup – intraperitoneal administration of L-NAME (40 mg/kg) during 7 weeks; IIc subgroup – intraperitoneal administration of L-NAME (40 mg/kg) during 4 weeks followed by 3 weeks of spontaneous recovery. 3) Group III, experimental group (seven rats) (intraperitoneal administration of L-NAME (40 mg/kg) for 7 weeks, and from the beginning of the fifth week (weeks 5-7), intraperitoneal administration of L-arginine (Sigma-Aldrich) (300 mg/kg) was added).
In control (group I) and experimental (group II (subgroups IIa, IIb and IIc) and group III) rats, parallel blood samples were drawn from the radial artery and brachial vein under anesthesia with 2% ether. At the end of the acute experiment, the chest was opened, and the middle part of the carotid artery was excised for morphological studies.
Blood pressure measurement
The blood pressure (systolic pressure (SP) and diastolic pressure (DP)) of the rats was measured every second day by the tail-cuff method by equipment “Systola” and oscillograph (“Neurobotics” LLC, Russia) according to the manufacturer’s protocol.
Measurement of total NO level in blood
The level of NOx in blood samples was determined by a modified method of Miranda et al. As the first step, blood serum sample deproteinization was achieved by adding equal volumes of 0.3 M NaOH to 100 µL of blood serum. It was mixed well and incubated for 5 min at room temperature. Then 100 µL of 5% ZnSO4 was added, mixed well and incubated for additional 5 min at room temperature. After the incubation, the mixture was centrifuged at 3,000 rpm at 4 °C for 15 min. An aliquot of 100 µL of the clear supernatant was then mixed with 200 µL of Griess reagent.
Griess reagent was prepared just prior to the assay and contained 0.25% VCl3, 0.1% sulfanilamide and 0.05% N-(1-naphthyl)-ethylenediamine (NED) (Sigma-Aldrich) in 0.5 M HCl. Reagent blank was the same but contained 100 µL of distilled water instead of the blood serum sample. The mixture was incubated for 30 min at 37 °C and absorbance was measured at 540 nm with a microplate reader (Multiclan GO, Thermo Fisher Scientific, Finland). The standard curve for NaNO2 was used to calculate total NOx concentration in the samples [13].
Morphological study
The carotid artery was cleaned and divided into proximal 1 mm long segments and immersed into the fixative (glutaraldehyde 3% in 0.1 M phosphate buffer) for a further 3 h; after washing in a phosphate buffer, the segments were fixed with 2% OsO4 in 0.1 M phosphate buffer. Then the specimens were stained with 2% uranyl acetate, dehydrated through ascending concentrations of alcohol and embedded in Durcupan ACM. Three randomly selected segments of the coronary artery were cut perpendicularly to the long axis. Both the inner circumference and arterial wall thickness (WT) were measured under light microscopy. The inner diameter (ID) and the WT (tunica intima and tunica media) of the carotid artery were calculated.
Statistical analysis
Statistical analysis of obtained results was performed by the use of the SPSS statistical analysis program package (version 10.0). The average parameters and their statistical derivations were analyzed. The difference between groups was evaluated by Student’s t-test. In all cases, statistical significance was obtained at P < 0.05. To establish the relationship between changes in blood NOx and pulse pressure (PP), Pearson’s correlation coefficients were calculated.
Results
L-NAME administration results
After 4 – 7 weeks of intraperitoneal administration of L-NAME (40 mg/kg) to rats (subgroups IIa and IIb), SP and DP increased by 81% and by 76%. Three weeks after the discontinuation of the 4-week intraperitoneal administration of L-NAME (spontaneous recovery), the indicators of SP and DP returned to the initial level (subgroup IIc) (Fig. 1). This means that L-NAME-induced experimental hypertension is reversible.
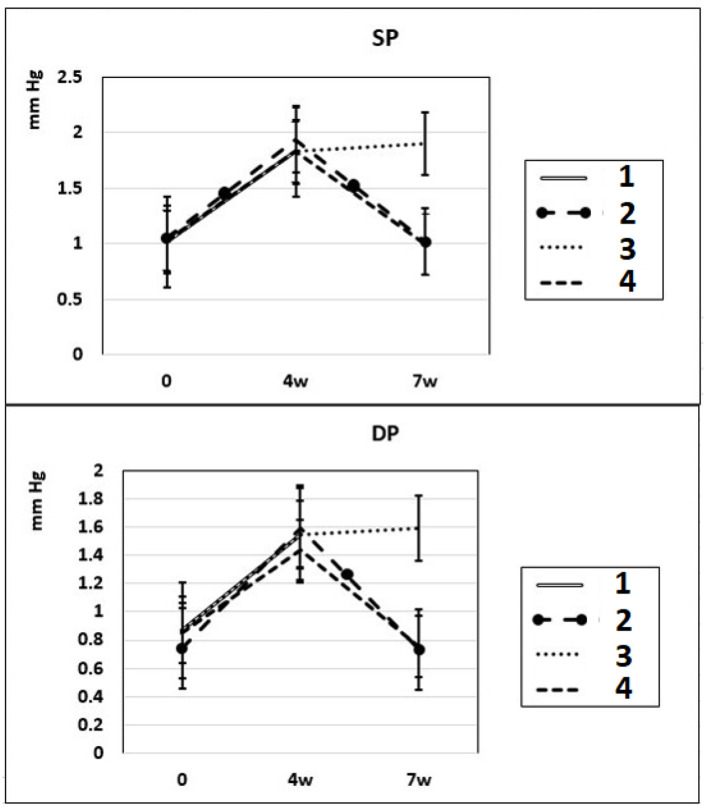
Dynamics of alterations in SP and DP in experimental rats. 1: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 4 weeks; 2: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 4 weeks followed by 3 weeks of spontaneous recovery; 3: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 7 weeks (4 + 3 weeks); 4: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 7 weeks, from the beginning of the fifth week (weeks 5 – 7), intraperitoneal administration of L-arginine (300 mg/kg) was added. SP: systolic pressure; DP: diastolic pressure; L-NAME: N-nitro-methyl ether-L-arginine.
In these experimental groups, the pulse rate had a tendency to increase (statistically insignificant) during 4 weeks of L-NAME administration (subgroup IIa) and stay at this level for the next 3 weeks of spontaneous recovery (subgroup IIc); after 7 weeks, pulse rate statistically significantly increased by 3% (P < 0.005) compared to the initial level (subgroup IIb). PP (the difference between SP and DP (SP – DP)) increased by 72% and 93% in subgroups IIa and IIb, respectively, and remained at this level after 3 weeks of discontinuation of L-NAME administration in rats (subgroup IIc) (Fig. 2). These data indicate that after 4 weeks of exposure to L-NAME, rats develop irreversible changes in vascular elasticity (stiffening).
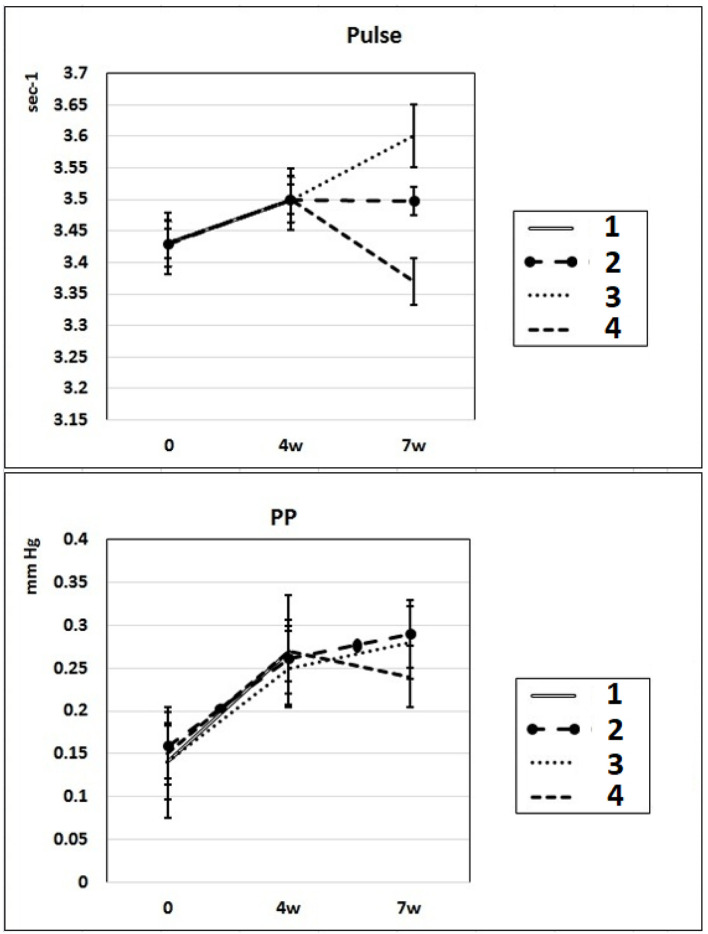
Dynamics of alterations of indicators of pulse rate and PP in experimental rats. 1: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 4 weeks; 2: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 4 weeks followed by 3 weeks of spontaneous recovery; 3: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 7 weeks (4 + 3 weeks); 4: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 7 weeks, from the beginning of the fifth week (weeks 5 – 7), intraperitoneal administration of L-arginine (300 mg/kg) was added. PP: pulse pressure; L-NAME: N-nitro-methyl ether-L-arginine.
In subgroup IIa (L-NAME administered for 4 weeks), the NO content decreased by 30% in the arterial blood and by 23% in the venous blood of rats compared to baseline. After 7 weeks of L-NAME treatment (subgroup IIb), the NO content in the arterial blood continued to decrease (by 18%) (Fig. 3). In subgroup IIc (3 weeks after discontinuation of 4 weeks of L-NAME administration), the NO content did not differ from the control level in either arterial or venous blood (Fig. 3).
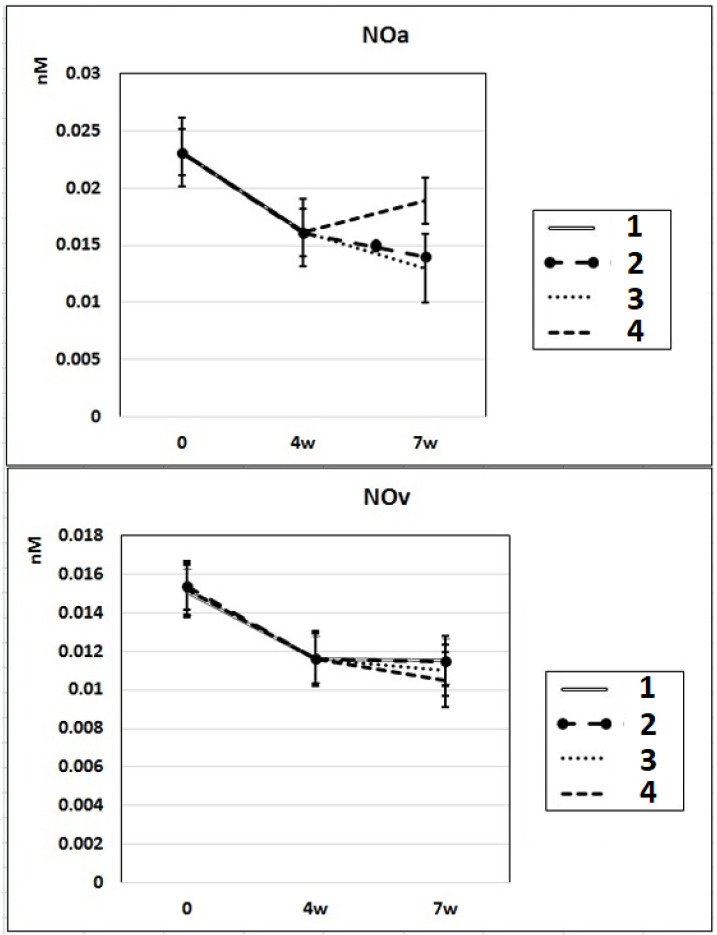
Dynamics of alterations of NO content in the rats’ arterial and venous blood. 1: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 4 weeks; 2: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 4 weeks followed by 3 weeks of spontaneous recovery; 3: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 7 weeks (4 + 3 weeks); 4: rats with intraperitoneal administration of L-NAME (40 mg/kg) for 7 weeks, from the beginning of the fifth week (weeks 5 – 7), intraperitoneal administration of L-arginine (300 mg/kg) was added. NO: nitric oxide; L-NAME: N-nitro-methyl ether-L-arginine.
L-arginine treatment results of L-NAME-induced hypertension
In rats of group III, where, against the background of 7 weeks of intraperitoneal administration of L-NAME (40 mg/kg) starting from the fifth week (weeks 5 – 7) for 3 weeks, they were treated with L-arginine (300 mg/kg), at the end of the experiment, the SP and DP indices returned to the initial level (Fig. 1), and the NO content in arterial blood increased by 29% compared with the value in animals untreated with L-arginine (Fig. 3). These data indicate that the combined administration of exogenous L-arginine with L-NAME provides restoration of NOS activity, which in turn contributes to an increase in the level of NO in arterial blood. In this experimental group (group III), the PP decreased by 18% compared with the values characteristic for subgroup IIb, which indicates an increase in the elasticity of the arterial walls (Fig. 2).
Morphological study results
The results of the morphometric evaluation of the geometry of the carotid artery showed that in control animals, the ID of the carotid artery was 825 ± 25.5 µm, and no significant alterations were found in the ID after treatment with L-NAME (subgroups IIa, IIb and IIc), or L-NAME plus L-arginine (group III) (Table 1). The WT (tunica intima + tunica media) of the carotid artery in control (group I) was 20.09 ± 0.85 µm; in animals treated with L-NAME (subgroups IIa, IIb and IIc), it was increased by 41-57%; after the additional treatment with L-arginine (group III), the WT of the carotid artery decreased by 27%.
Table 1
Morphometric Parameters of Carotid Artery
| Groups | ID (µm) | WT (µm) |
|---|---|---|
| Group I | 825 ± 25.5 | 21.39 ± 0.85 |
| Group IIa | 832 ± 22.6 | 30.21 ± 0.89* |
| Group IIb | 829 ± 23.0 | 31.54 ± 0.85* |
| Group IIc | 828 ± 26.7 | 33.53 ± 3.41* |
| Group III | 827 ± 24.5 | 24.56 ± 2.45** |
*P < 0.01 vs. controls. **P < 0.01 vs. L-NAME. ID: inner diameter; WT: wall thickness; L-NAME: N-nitro-methyl ether-L-arginine.
Author information
aDepartment of Physics, Biophysics, Biomechanics and Informational Technologies, Tbilisi State Medical University, Tbilisi, Georgia
bDepartment of Cardiology, Amtel Hospital, Tbilisi, Georgia
cDepartment of Pathology, Faculty of Medicine, I. Javakhishvili Tbilisi State University, Tbilisi, Georgia
dI. Beritashvili Center of Experimental Biomedicine, Tbilisi, GeorgiaeEuropean University, Tbilisi, Georgia
fCorresponding Author: Tamar Sanikidze, Department of Physics, Biophysics, Biomechanics and Informational Technologies, Tbilisi State Medical University, 33 Vaja Pshavela Av., Tbilisi 380060, Georgia.