Effect of Complex Weight-Reducing Interventions on Rhythm Control in Obese Individuals with Atrial Fibrillation Following Catheter Ablation: A Study Protocol
Abstract
Introduction
Obesity and atrial fibrillation (AF) pose a significant burden on healthcare systems worldwide. Reduction of body weight has been documented to reduce the risk of AF. Little is known about the effect of different weight-reducing interventions including bariatric surgery in obese individuals on the risk of arrhythmia recurrence following catheter ablation (CA) for AF, and about the pathophysiological mechanisms linking these two conditions.
Methods
The Effect of complex weigHt-reducing interventiOns on rhythm control in oBese subjects wITh Atrial Fibrillation (HOBIT-AF) is a single-blinded, parallel-group randomised controlled trial with 18-month follow-up to assess the effect of complex weight-reducing interventions supported by the use of smart technologies and bariatric surgery on the arrhythmia burden in obese individuals following CA for AF. One hundred and sixty individuals (age 18–70 years, body mass index ≥ 30 kg/m2) will be randomised in a 1:1 fashion to undergo a structured weight reduction programme and sleeve gastrectomy (when indicated and preferred by the patient) aiming to achieve greater than 10% weight reduction from baseline (intervention group) or standard post-ablation medical care (control group). Two-week continuous ECG monitoring will be used 3 and 18 months after CA to assess the arrhythmia burden. Other investigations will include transthoracic echocardiography with quantification of epicardial adipose tissue, and markers of low-grade inflammation and circulating adipokines.
Planned Outcomes
The main objective is to assess the effect of complex weight-reducing interventions on the arrhythmia burden and quality of life. Subgroup analyses to identify patient subgroups preferentially benefiting from weight loss related to a decrease in arrhythmia burden will be performed. Exploratory objectives will include investigation of potential mechanisms linking weight reduction with amelioration of arrhythmia burden such as changes in markers of low-grade inflammation, circulating adipokines, cytokines, monocytes or reduction of epicardial adipose tissue volume.
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13862834
Introduction
Obesity represents a major public health challenge because it substantially increases the risk of multiple medical conditions including type 2 diabetes, fatty liver disease, arterial hypertension, myocardial infarction, stroke, dementia and several types of cancers [1]. Its prevalence has been on the rise over the last decades and has currently reached a stage of a pandemic. According to the World Health Organization obesity currently affects over 650 million people worldwide and its prevalence is estimated to rise further [2]. Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia with approximately 5.2 million people affected solely in the USA and its prevalence is estimated to increase threefold in the next three decades [3–5]. Globally, direct and indirect costs linked with obesity and AF reach hundreds of billions of US dollars per annum, thus posing a substantial economic burden on healthcare systems worldwide [6, 7].
The links between obesity and AF have been established, yet there is much more we need to understand to be able to tackle these conditions more effectively. Obesity represents the second strongest population-attributable risk factor for AF besides arterial hypertension with almost every fifth case of AF linked with overweight or obesity [8]. Incremental increases in body mass index (BMI) are associated with a significant excess risk of AF in different clinical settings. For every 5-unit increase in BMI there is a 19–29% additional risk of incident AF, a 10% additional risk of post-operative AF and a 13% additional risk of post-ablation AF [9]. In another meta-analysis, obese individuals showed a 38% increase in post-ablation AF recurrence when compared to a group with normal weight [10]. Conversely, significant weight loss achieved by bariatric surgery was found to reduce the risk of new-onset AF by 29% in the prospective matched cohort Swedish Obese Study [11]. Among patients with symptomatic AF, weight reduction achieved by a structured weight management programme resulted in a reduction of AF symptom burden and severity, the number of episodes and cumulative AF duration [12]. Similarly, in the 5-year-long follow-up study LEGACY, weight loss of greater than 10% resulted in a sixfold greater probability of arrhythmia-free survival when compared to patients with modest or no weight change at all [13].
Pathophysiological mechanisms linking obesity and AF are highly complex and only incompletely understood. Except for the established factors associated with AF such as atrial dilation, increased left ventricular diastolic filling pressure and neurohumoral activation, novel mechanisms related to systemic low-grade inflammation and production of adipose tissue-derived factors (adipokines) have emerged [14, 15]. Obesity is associated with increased production and circulatory levels of pro-inflammatory cytokines including tumour necrosis factor alpha (TNFα), interleukin-6 (IL-6), interferon gamma (INFγ) and adipokines such as leptin, adiponectin and resistin [16–19]. Conversely, weight reduction achieved by laparoscopic sleeve gastrectomy can lead to improvement of pro-inflammatory profile in subcutaneous fat [20]. Many studies have demonstrated a strong correlation between inflammatory markers C-reactive protein (CRP), TNFα and IL-6 and the presence and duration of AF [21, 22]. A recent study showed decreased levels of apolipoprotein A1, the principal protein component of high-density lipoprotein cholesterol with anti-inflammatory and antioxidant properties, in patients with AF when compared to non-AF controls [23]. Moreover, the volume of adipose tissue (in particular in the areas overlying the atria) plays a role in potentiating local inflammation [24] and was shown to be a better predictor of future risk of AF than BMI, and correlated strongly with levels of pro-inflammatory markers [25–27].
Catheter ablation (CA) is a well-established treatment modality for the prevention of AF recurrences [28]. Some studies have documented improved outcomes of CA in patients after aggressive risk factor management, including weight reduction [29]. However, more in-depth analysis in the form of a randomised controlled trial of the impact of different types of weight-reducing interventions on AF burden after CA is still missing. Calculating the proportion of time spent in AF or associated atrial tachycardia (AT) during the monitoring period is the most recommended way of assessing the arrhythmia burden [30], but it requires prolonged ambulatory Holter ECG monitoring which is time-consuming for investigators and difficult for patients. Studies listed above assessed the risk of AF either by establishing the risk of new-onset AF [11] or by the help of the Atrial Fibrillation Severity Scale (AFSS) [12, 13, 31], whilst data from a 7-day Holter ECG monitoring period served only as a secondary outcome in some [12, 13]. Thus, there is an unmet need for data from longer monitoring periods to better characterise the effect of weight loss achieved via different weight-reducing strategies including bariatric surgery on subsequent risk of recurrent AF/AT.
Hypotheses
We hypothesise that obese individuals following CA for AF who will achieve a sustained weight reduction of greater than 10% due to enrolment in the complex programme of weight-reducing interventions will manifest significantly decreased AF/AT burden and improved quality of life as compared to individuals who receive standard post-ablation medical care. We further hypothesise that change in AF/AT burden will correlate with the reduction of body weight irrespective of the treatment modality.Go to:
Methods
Study Design
The Effect of complex weigHt-reducing interventiOns on rhythm control in oBese subjects wITh Atrial Fibrillation (HOBIT-AF, study ID number NU20-02-00190) is a single-blinded, parallel-group randomised controlled trial. This study will enrol obese individuals who recently underwent CA for AF and are motivated to reduce their body weight. This study has been registered on US National Library of Medicine ClinicalTrials.gov database (NCT04560387). The study protocol was approved by the institutional ethics committee. This study will be conducted in accordance with the Helsinki Declaration of 1964 and its later amendments and the ICH Good Clinical Practice Guidelines.
Study Population
One hundred and sixty individuals will be recruited following the provision of signed informed consent. Inclusion criteria are 18–70 years of age, currently undergoing CA for paroxysmal or short-term persistent AF and BMI ≥ 30 kg/m2. To minimise the influence of CA strategy on the subsequent recurrences of AF/AT, all subjects will undergo isolation of the pulmonary veins only. Exclusion criteria will comprise a history of CA for AF, myocardial infarction, stroke or pulmonary embolism less than 3 months before enrolment, left ventricular ejection fraction less than 40%, left atrium volume index greater than 60 ml/m2, active thyroid disease, chronic kidney disease stage IV–V (estimated glomerular filtration rate less than 30 ml/min/1.73 m2), chronic liver disease, active malignancy or inability to comply with study procedures.
Enrolment and Randomisation
CA is a necessary prerequisite for inclusion into the study and as such is not considered as a study intervention. Indications for this procedure are based on the current European Society of Cardiology clinical guidelines for diagnosis and management of AF [32]. Following CA, eligible participants who are willing to take part in the trial will be asked to sign a written informed consent. Participants will be randomised in a 1:1 fashion into either the intervention group or standard post-ablation medical care (control group). Participants in the intervention group will undergo a structured weight reduction programme and sleeve gastrectomy (when indicated and preferred by the patient) aiming to achieve greater than 10% weight reduction from baseline. Participants randomised into the standard post-ablation medical care (control group) will be offered participation in the structured weight reduction programme once the trial is finished as a further incentive to take part in the trial. The enrolment period of the trial will last 24 months and each participant will be followed for 18 months (Fig. 1).
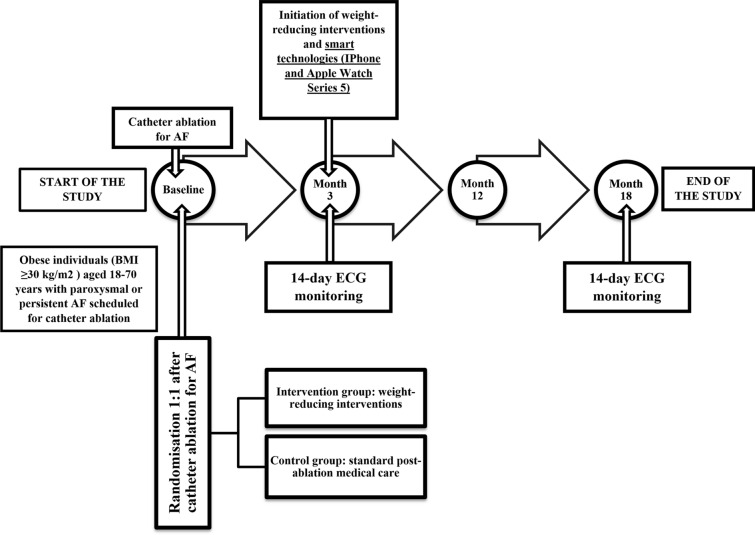
Study flowchart. Altogether four study visits are planned: baseline and months 3, 12 and 18. AF atrial fibrillation, BMI body mass index, ECG electrocardiogram
Study Interventions
The complex programme of weight-reducing interventions including education, diet counselling, supportive pharmacotherapy, and regular physical activity is aimed at achieving and maintaining at least 10% reduction of baseline body weight. This structured motivational and goal-directed programme will be physician-led and will also involve nutritionists, educators, psychologists and physiotherapists. Initially, based on the input data provided by the patient, an individual nutritional plan will be designed to reduce caloric intake by 10%. Low-intensity aerobic exercise for 30 min will be prescribed three times a week to increase the intensity (Borg scale 11–14) and frequency to five times a week and participants will be offered the possibility to participate in regular physiotherapist-led group exercises. Participants will be required to maintain a diet and physical activity diary. Regular reviews will be scheduled for every 3–6 months according to the actual weight loss.
The HOBIT-AF trial will employ smart technologies to increase the motivation and adherence to introduced lifestyle changes. Participants in the intervention group will be provided with an iPhone and Apple Watch Series 5 (both by Apple Inc, Cuppertino, CA, USA) at month 3 visit (Fig. 1) for the rest of the trial. A purpose-made mobile application IKEM Online Fit will be pre-installed on iPhone devices and those with own iPhones will be asked to download the application from the Apple App Store. This application will enable participants to generate an ECG report in case of an arrhythmia episode. Participants will be asked to regularly provide their weight, blood pressure and physical activity, and these data, together with heart rate, ECG trace and steps count will be securely transferred to investigators at the local home-monitoring centre. Besides, a custom-made website was designed for the participants of the trial containing useful tips concerning nutrition, cooking tips and physical activities.
Bariatric surgery (sleeve gastrectomy) will be offered to those fulfilling the current indication criteria for bariatric surgery in the Czech Republic (BMI > 35 kg/m2). All procedures associated with surgery as well as the surgery itself will be performed at an external surgical department. Sleeve gastrectomy is the bariatric procedure of choice as it is currently the most frequently performed restrictive type of bariatric intervention with proven efficacy on weight reduction, metabolic status and low-grade inflammation [33].
Follow-up Management
A total of four visits are planned during the study period (Fig. 1): enrolment/baseline/randomisation visit (at the time of CA), therapy initiation visit at month 3 (end of post-ablation blanking period), and two follow-up visits at month 12 and month 18.
Arrhythmia management will be adjusted to minimise the interference with study interventions and/or investigations. Antiarrhythmic drugs will be prescribed or discontinued before entering the active phase of the trial (month 3) depending on whether the patient will or will not be considered at risk of arrhythmia recurrence. This will be done at the discretion of investigators and the medical therapy will not be modified during the trial unless clinically indicated. In all patients, the strategy of rhythm control will be applicable throughout the trial. In case of recurrence of persistent AF/AT, electrical cardioversion will be performed without undue delay. On the other hand, re-ablation procedures will be postponed until the end of the trial (month 18) if clinically acceptable.
Study Investigations
A timetable of study investigations is provided in Table 1. At each visit medical history, anthropometric measures including body weight and height, BMI, waist circumference, and body composition estimation by bioelectrical impedance analysis will be obtained. Blood sampling will be performed to analyse the following parameters: urea, creatinine, electrolytes, liver enzymes, albumin, bilirubin, uric acid, lipid profile, full blood count, clotting screen, thyroid-stimulating hormone (TSH), oestradiol, testosterone, glycated haemoglobin (HbA1c), natriuretic peptides (ANP, BNP), serum parameters of low-grade inflammation (high sensitivity CRP, TNFα, INFγ, IL-1, 6, 8, 10, IL-1 receptor antagonist, monocyte chemoattractant protein 1), circulating adipokines (leptin, adiponectin, omentin, resistin, chemerin, fibroblast growth factor 21), circulating monocytes, mRNA expression of inflammatory cytokines and chemokines. Oral glucose tolerance test will be performed in non-diabetic participants at month 3 and month 18. Urinalysis will include urine sediment examination, chemical analysis and evaluation of the albumin/creatinine ratio.
Table 1
Study investigations
| Investigations | Baseline | Month 3 | Month 12 | Month 18 |
|---|---|---|---|---|
| Written informed consent | X | |||
| Medical history | X | X | X | X |
| Anthropometric measures | X | X | X | X |
| Venous blood sampling | X | X | X | X |
| 14-day ECG monitoring | X | X | ||
| Transthoracic echocardiography | X | X | ||
| Screening for sleep apnoea | X | |||
| Spiroergometry | X | X | ||
| Screening for diabetic microvascular complications | X | |||
| Quality of life questionnaires | X | X | X | X |
All participants will be asked to fill in the Minnesota Living With Heart Failure Questionnaire (MLWHFQ), Atrial Fibrillation Effect on QualiTy-of-life Questionnaire (AFEQT) and the Short Form Health Survey (SF-12) at each study visit.
Other investigations will include screening for obstructive sleep apnoea, spiroergometry and screening for microvascular diabetic complications (peripheral and autonomic neuropathy, retinopathy and nephropathy) in a subgroup of participants with diabetes.
Two sessions of 14-day ECG monitoring periods will be performed at month 3 and month 18 by an independent telemedicine company that will periodically provide ECG loop recorders to all participants and will be responsible for the blinded and accurate AF/AT burden analysis.
A standard transthoracic echocardiography examination including epicardial adipose tissue volume assessment will be performed at month 3 and month 18.
Study Objectives
The primary objective is the change (either absolute or relative) in AF/AT burden between month 3 visit (initiation of the therapy) and month 18 visit (end of the trial). AF/AT burden itself will be derived from 14-day Holter monitoring and expressed as a sum of the duration of all AF/AT episodes divided by total monitoring time. There are three secondary objectives: (1) arrhythmia burden (‘days with arrhythmia’) in the last 6 months of the follow-up (between month 12 and 18), as assessed by medical records and patients’ diaries, (2) the change in the quality of life between month 3 and month 18 visit and (3) the change in epicardial adipose tissue volume between baseline and month 18 visit. All these objective measures will be compared between the main study groups composed by random allocation. Other objectives are correlations of (1) change in AF/AT burden and (2) change in the quality of life with the magnitude of body weight reduction irrespective of the treatment modality. We will also aim to identify patient subgroups preferentially benefiting from weight loss in relation to AF/AT burden reduction: male vs. female, people with and without diabetes, individuals with higher vs. lower degree of obesity (BMI > 40 vs. BMI 30–35 kg/m2). Exploratory objectives include identification of potential mechanisms linking the reduction of adipose mass and improving the arrhythmia outcome. This will be done by correlation analysis between the change in AF/AT burden and the change in markers of low-grade inflammation, circulating adipokines and reduction of epicardial adipose tissue volume.
Sample Size Calculation and Statistical Analysis
Sample size calculation is based on the study by Abed et al. in which weight-reducing lifestyle interventions were associated with a 68.2% (1055 min) difference of AF burden in comparison to non-interventional controls. Given these data, a sample size of 68 individuals in each group will give 80% power of detecting a difference in AF burden as statistically significant at the 5% two-sided significance level. Assuming a dropout rate of approximately 20%, we plan to enrol 160 (80 + 80) participants in total. Statistical analysis will be performed using SigmaStat software (Systat Software Inc., San Jose, CA, USA). Results will be expressed as mean ± SEM. We will use t tests or rank sum tests for two-group comparisons and one-way ANOVA or Kruskal–Wallis test for multiple group comparisons as appropriate according to the normality of data distribution. A P value less than 0.05 will be considered statistically significant.
Strengths and Limitations
The strengths of this study include the randomised design, maximised obesity intervention due to the availability of bariatric surgery, and, at least in part, objective assessment of the arrhythmia outcome. The two 14-day long ECG monitoring periods will provide investigators with a sufficient amount of data enabling a relatively accurate assessment of AF/AT burden which is a robust and recommended clinical endpoint. The trial is limited by the heterogeneity of intervention because only some participants randomised into the intervention group will be eligible for sleeve gastrectomy and, in addition, some of these will not undergo this procedure because of their personal preferences. Yet, we feel that this cannot be avoided as sleeve gastrectomy is an irreversible surgical intervention with serious consequences. We, therefore have to fully respect patients’ autonomy concerning such important decisions about their life and health. Another limitation is the single-blinded design of the trial. However, the main intervention cannot be principally performed double-blinded, and even single-blinding is not fully guaranteed for all study investigations. Furthermore, the majority of investigations in the trial are based on objective measures that limit the subjective bias of non-blinded participants.
Conclusions
The HOBIT-AF trial is the first randomised controlled trial to assess the effect of complex weight-reducing interventions and bariatric surgery on the arrhythmia burden in obese individuals with recent catheter ablation for AF. The design of the trial and its primary, secondary and exploratory endpoints have the potential to broaden our understanding of the causative factors linking obesity and arrhythmia and might open up new therapeutic pathways for combatting the current AF and obesity epidemics.