Emergency department thoracotomy for the critically injured patient: Objectives, indications, and outcomes
Release pericardial tamponade and control cardiac hemorrhage
The highest survival rate following EDT is in patients with penetrating cardiac wounds, especially when associated with pericardial tamponade [7,17]. Early recognition of cardiac tamponade, prompt pericardial decompression, and control of cardiac hemorrhage are the key components to successful EDT and patient survival following penetrating wounds to the heart [24]. The egress of blood from the injured heart, regardless of mechanism, results in tamponade physiology. The classic description of clinical findings, Beck’s triad, is rarely observed in the emergency department; therefore, a high index of suspicion in the at-risk patient sustaining penetrating torso trauma is crucial, with prompt intervention essential. The first two phases of cardiac tamponade – restricted ventricular diastolic filling, compromised stroke volume and coronary perfusion, and diminished cardiac output – may be aggressively managed with definitive airway control, volume resuscitation to increase preload, and pericardiocentesis. The patient in the third phase of tamponade – intrapericardial pressure approaches the ventricular filling pressure with profound hypotension (SBP < 60) – should undergo EDT rather than pericardiocentesis as the management for evacuation of pericardial blood [25,26]. Following release of tamponade, the source of tamponade can be directly controlled with appropriate interventions based on the underlying injury.
Control intrathoracic hemorrhage and perform open cardiac massage
Life-threatening intrathoracic hemorrhage occurs in less than 5% of patients following penetrating injury presenting to the ED, and in even lower percentage of patients sustaining blunt trauma [27]. The most common injuries include penetrating wounds to the pulmonary hilum and great vessels; less commonly seen are torn descending thoracic aortic injuries with frank rupture or penetrating cardiac wounds exsanguinating into the thorax through a traumatic pericardial window. There is a high mortality rate in injuries to the pulmonary or thoracic great-vessel lacerations due to the lack of hemorrhage containment by adjacent tissue tamponade or vessel spasm. Either hemithorax can rapidly accommodate more than half of a patient’s total blood volume before overt physical signs of hemorrhagic shock occur. Patients with exsanguinating wounds require EDT with rapid control of the source of hemorrhage if they are to be salvaged.
In patients with cardiopulmonary arrest, external chest compression provides approximately 20 to 25% of baseline cardiac output, with 10 to 20% of normal cerebral perfusion [28-30]. While this degree of vital organ perfusion can provide reasonable salvage rates for 15 minutes, few normothermic patients survive 30 minutes of closed-chest compression. Moreover, in models of inadequate intravascular volume (hypovolemic shock) or restricted ventricular filling (pericardial tamponade), external chest compression fails to augment arterial pressure or provide adequate systemic perfusion; the associated low diastolic volume and pressure result in inadequate coronary perfusion [31]. Therefore, closed cardiac massage is ineffective for postinjury cardiopulmonary arrest. The only potential to salvage the injured patient with ineffective circulatory status is immediate EDT.
Achieve thoracic aortic cross clamping
The rationale for temporary thoracic aortic occlusion in the patient with massive hemorrhage is two-fold. First, in patients with hemorrhagic shock, aortic cross clamping redistributes the patient’s limited blood volume to the myocardium and brain [9]. Second, patients sustaining intraabdominal injury may benefit from aortic cross clamping due to reduction in subdiaphragmatic blood loss [8]. Temporary thoracic aortic occlusion augments aortic diastolic and carotid systolic blood pressure, enhancing coronary as well as cerebral perfusion [32,33]. Experimental observations suggest that temporary aortic occlusion is valuable in the patient either with shock due to non-thoracic trauma or in patients with continued shock following the repair of cardiac or other exsanguinating wounds [34,35]. Indeed, occlusion of the descending thoracic aorta appears to increase the return of spontaneous circulation following cardiopulmonary resuscitation [36,37]. Reports of successful resuscitation using EDT in patients in hemorrhagic shock and even sustaining cardiac arrest following extremity and cervical injuries exist [38]. In these situations, EDT may be a temporizing measure until the patient’s circulating blood volume can be replaced by blood product transfusion. In carefully selected patients, aortic cross clamping may effectively redistribute the patient’s blood volume until external replacement and control of the hemorrhagic source is possible. However, once the patient’s blood volume has been restored, the aortic cross clamp should be removed, as there is substantial metabolic cost and a finite risk of paraplegia associated with the procedure [39-41]. Typically, complete removal of the aortic cross clamp or replacement of the clamp below the renal vessel should be performed within 30 minutes.
Evacuate bronchovenous air embolism
Bronchovenous air embolism can be a subtle entity following thoracic trauma, and is likely to be much more common than is recognized [42-44]. The clinical scenario typically involves a patient sustaining penetrating chest injury who precipitously develops profound hypotension or cardiac arrest following endotracheal intubation and positive-pressure ventilation. Traumatic alveolovenous communications produce air emboli that migrate to the coronary arterial systems; any impedance in coronary blood flow causes global myocardial ischemia and resultant shock. The production of air emboli is enhanced by the underlying physiology – there is relatively low intrinsic pulmonary venous pressure due to associated intrathoracic blood loss and high bronchoalveolar pressure from assisted positive pressure ventilation. This combination increases the gradient for air transfer across bronchovenous channels [45]. Although more often observed in penetrating trauma, a similar process may occur in patients with blunt lacerations of the lung parenchyma.
Immediate thoracotomy with pulmonary hilar cross clamping prevents further propagation of pulmonary venous air embolism. Thoracotomy with opening of the pericardium also provides access to the cardiac ventricles; with the patient in the Trendelenburg’s position (done to trap air in the apex of the ventricle), needle aspiration is performed to remove air from the cardiac chambers. Additionally, vigorous cardiac massage may promote dissolution of air already present in the coronary arteries [44]. Aspiration of the aortic root is done to alleviate any accumulated air pocket, and direct needle aspiration of the right coronary artery may be lifesaving.
Technical pearls
The optimal benefit of EDT is achieved by a surgeon experienced in the management of intrathoracic injuries. The emergency physician, however, should not hesitate to perform the procedure in the moribund patient with a penetrating chest wound when thoracotomy is the only means of salvage. The technical skills needed to perform the procedure include the ability to perform a rapid thoracotomy, pericardiotomy, cardiorrhaphy, and thoracic aortic cross clamping; familiarity with vascular repair techniques and control of the pulmonary hilum are advantageous. As the procedure has been described in detail elsewhere, we will briefly touch on some “pearls” which may facilitate one’s approach and success in performing EDT.
Thoracic incision
Upon patient arrival and determination of the need for EDT, the patient’s left arm should be placed above the head to provide unimpeded access to the left chest. The thoracotomy incision should start on the right side of the sternum; if sternal transection is required, this saves the time consuming step of performing an additional skin incision (Figure (Figure2).2). As the initial incision is carried transversely across the chest, as one passes beneath the nipple a gentle curve in the incision toward the patient’s axilla rather than direct extension to the bed should be performed; this curvature in the skin correlates with the natural curvature of the rib cage. The initial execution of a clamshell thoracotomy should be done in hypotensive patients with penetrating wounds to the right chest. This provides immediate, direct access to a right-sided pulmonary or vascular injury while still allowing access to the pericardium from the left side for open cardiac massage. Clamshell thoracotomy may also be considered in patients with presumed air embolism, providing access to the cardiac chambers for aspiration, coronary vessels for massage, and bilateral lungs for obliteration of the source. Once the right pleural space is opened, the rib retractor should be moved to more of a midline position to enhance separation of the chest wall for maximal exposure. When visualization of penetrating wounds in the aortic arch or major branches is needed, the superior sternum is additionally split in the midline. If the sternum is divided transversely, the internal mammary vessels must be ligated when perfusion is restored; this may be performed using either a figure of eight suture with 2-0 silk or by clamping the vessel with a tonsil and individually ligating it with a 2-0 silk tie.
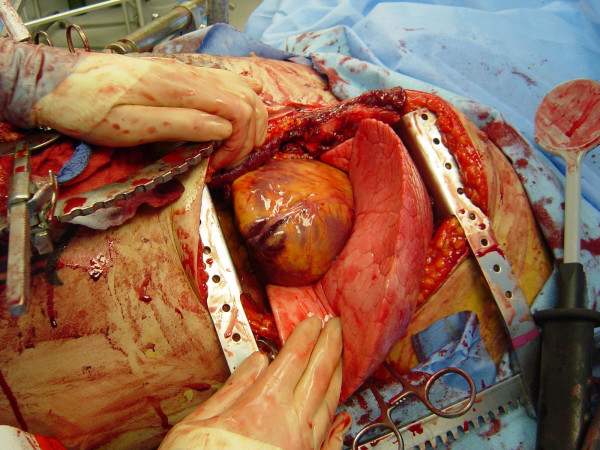
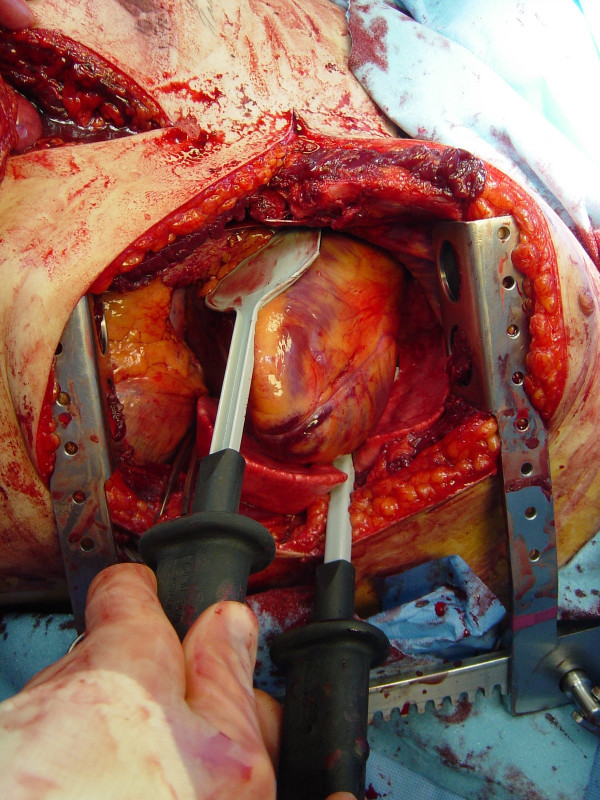
Pericardiotomy and cardiac hemorrhage control
If the pericardium is not tense with blood it may be picked up at the apex with toothed forceps and sharply opened with scissors. If tense pericardial tamponade exists, a knife or the sharp point of a scissors is often required to initiate the pericardiotomy incision. Prompt hemorrhage control is paramount for a cardiac injury. In the beating heart, cardiac bleeding sites should be controlled immediately with digital pressure on the surface of the ventricle and partially occluding vascular clamps on the atrium or great vessels. Efforts at definitive cardiorrhaphy may be delayed until initial resuscitative measures have been completed. In the nonbeating heart, cardiac repair is done prior to defibrillation and cardiac massage. Cardiac wounds in the thick walled left ventricle are best repaired with 3-0 non-absorbable running or horizontal mattress sutures. Buttressing the suture repair with Teflon pledgets is ideal for the thinner right ventricle. When suturing a ventricular laceration, care must be taken not to incorporate a coronary vessel into the repair. In these instances, vertical mattress sutures should be used to exclude the coronary and prevent cardiac ischemia (Figure (Figure3).3). In the more muscular left ventricle, particularly with a linear stab wound, control of bleeding can often be temporized with a skin-stapling device. Low-pressure venous, atrial, and atrial appendage lacerations can be repaired with simple running or pursestring sutures. Use of a foley catheter for temporary occlusion of cardiac injuries has been suggested; in our experience this may inadvertently extend the injury due to traction forces.
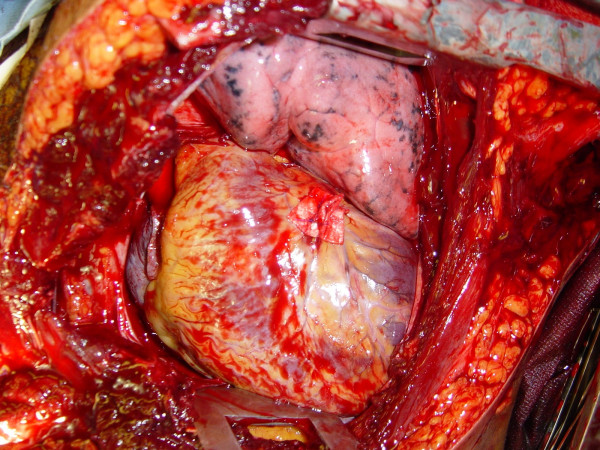
Advanced cardiac life support interventions including cardiac massage
The restoration of organ and tissue perfusion may be facilitated by a number of interventions. Dysrhythmias should be treated according to current guidelines [46] and internal defibrillation has similar indications as closed chest CPR. Familiarity with the internal cardiac paddles and appropriate charging dosages in joules is required (Figure (Figure4).4). In the event of cardiac arrest, bimanual internal massage of the heart should be instituted promptly. We prefer to do this with a hinged clapping motion of the hands, with the wrists apposed, sequentially closing from palms to fingers. The ventricular compression should proceed from the cardiac apex to the base of the heart. The two-handed technique is strongly recommended, as the one-handed massage technique poses the risk of myocardial perforation with the thumb. Pharmocologic adjuncts to increase coronary and cerebral perfusion pressure may be needed; the first agent in resuscitation at this juncture is intracardiac epinephrine. Epinephrine should be administered using a specialized syringe, which resembles a spinal needle, directly into the left ventricle. Typically the heart is lifted up slightly to expose the more posterior left ventricle, and care is taken to avoid the circumflex coronary during injection.