Management of Massive Grain Aspiration
Case Report
While working, a previously healthy 16-yr-old, 65-kg boy fell into a 3-m deep transport wagon being filled with whole wheat grain and was immediately submersed. Efforts to rescue him required approximately 5–7 min. He was unresponsive, cyanotic, and making spontaneous respiratory efforts on removal. During transfer by ambulance to a peripheral hospital, ventilation was attempted with oxygen via a self-inflating bag and mask, but there was no obvious gas exchange due to the extreme lack of compliance of his respiratory system. On arrival 40 min after the accident, he was unresponsive with equal pupils (approximately 4–5 mm diameter) that reacted slowly to light. Pulse was 120 beats/min; blood pressure was 170/80 mmHg, and he was making shallow respiratory efforts at a rate of 25–30 breaths/min. An intravenous catheter was placed, and the patient was intubated with a 7.5-mm endotracheal tube (ETT) after his mouth and pharynx were cleared of large amounts of packed grain by manual extraction, suction, and forceps during direct laryngoscopy. It was possible to advance the cuff of the ETT to just below the level of the vocal cords, but the ETT would not pass further probably due to impacted grain in the trachea. Ventilation was attempted with oxygen using a self-inflating bag via the ETT, but no effective tidal volumes could be detected by auscultation or inspection during positive pressure ventilation. Arterial blood gases (ABG) at that time revealed a severe hypercapnia and a mixed respiratory and metabolic acidosis. (Table 1). He received intravenous hydrocortisone, 1 g, and sodium bicarbonate, 88 mEq, and was transferred to the university hospital by ambulance.
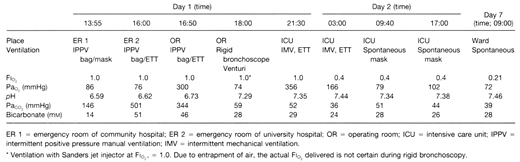
On arrival in the emergency department 2 h 40 min after the accident, his neurologic status was unchanged; blood pressure was 140/80; pulse was 110 beats/min; saturation by pulse oximetry (SpO2) was 98%, and he was making shallow gasping respiratory efforts. Subcutaneous emphysema was noticed in the supraclavicular areas bilaterally. He was treated with a bolus of intravenous sodium thiopental, 300 mg, for possible cerebral protection in case of subsequent hypoxemia during bronchoscopy. Pancuronium, 5 mg, was administered intravenously; albuterol, 400 micro gram, was administered via the ETT using a metered dose inhaler, and the ETT was changed to a 9.0-mm inner diameter tube. A blood sample showed extreme hypercapnia and acidosis (see Table 1). Manual ventilation remained extremely difficult, requiring high inspiratory pressures that generated almost no detectable tidal volumes.
The Thoracic Surgery service was called, and an attempt was made at rigid bronchoscopy in the emergency room. A 9-mm rigid ventilating bronchoscope (Karl Storz GmbH and Co., Tuttlingren, Germany) was passed into the trachea, which was found to be packed with grains of wheat. The patient desaturated rapidly to 75% SpO2. Ventilation with oxygen during rigid bronchoscopy was attempted by manual ventilation via the lumen of the bronchoscope and by Sanders jet ventilation via the lumen of the bronchoscope, [2 ] but oxygenation could not be maintained. The patient was reintubated, and the SpO2returned to 98% after 5 min of manual self-inflating bag ventilation. Rigid bronchoscopy was repeated using apneic oxygenation three times during the next 30 min. Each time 5–10 wheat seeds were removed using alligator-grasping forceps. Each time, the patient desaturated precipitously and required reintubation and 5–10 min of manual ventilation to restore the saturation. It became obvious that there was so much grain in the tracheobronchial tree that this bronchoscopic technique was not feasible, and a decision was made to perform the rigid bronchoscopy while using femoral-femoral cardiopulmonary bypass for oxygenation. The patient was transferred to the operating room where noninvasive blood pressure, electrocardiograph (ECG), and SpO2monitors were applied. Intravenous sufentanil, 200 micro gram, and midazolam, 4 mg, were administered. The end-tidal CO2concentration was > 100 mmHg, which exceeded the limits of the end-tidal CO2monitor (Nellcor, N-1000 Nellcor Inc., Hayward, CA). Due to the high airway resistance, it was impossible to maintain the SpO2> 85% despite an FiO2of 1.0 using the ventilator on the anesthetic machine (Nuffield-400, Penlon Inc., Abingdon, UK) or the manual anesthetic ventilation circuit, which have high airway pressure release valves preset at 60 cm H2O. Satisfactory SpO2levels (> 90%) were restored only with self-inflating bag ventilation with oxygen using extremely forceful manual compressions.
Before the proposed emergency cardiopulmonary bypass, a radial arterial line and a 8.5-French subclavian central venous catheter were placed for monitoring and vascular access. When the patient was placed in the Trendelenburg position for central line placement, it was noted that after each positive pressure inflation, several grain seeds would fall to the proximal end (now the most dependent portion) of the ETT during expiration.
It became evident that the more vigorous the manual inflation efforts, the more seeds fell into the ETT. These could then be removed by disconnecting the bag from the ETT and shaking the proximal end of the ETT. It was then discovered that manual percussion (clapping) of the patient’s chest caused even more grain to be dislodged with each breath. During the subsequent 20 min, the surgeon performed manual chest percussions as the patient was moved alternately from supine to lateral and prone positions. An estimated 200 ml of wheat grain was removed by this method. Clinically, ventilation became progressively easier. After removal of all possible grain by this method, it was then possible to perform rigid bronchoscopy and maintain SpO2with Sanders jet ventilation while the trachea and major bronchi were cleared of grain with alligator forceps.
Finally, all visible grains were removed from the subsegmental bronchi with suction extraction using a 6-mm fiberoptic bronchoscope (FOB;Figure 1) via a 9.0-mm ETT with swivel adapter. This procedure required direct application of the distal end of the FOB to each wheat grain. Approximately 150 further grains of wheat were removed by this method. Total operating room time was 4 h.
Figure 1. An enlarged photograph showing several of the wheat grains that were removed by bronchoscopy and the distal end of the 6.0-mm diameter fiberoptic bronchoscope.

The patient was transferred to the SICU intubated and initially ventilated with FiO2, 1.0; tidal volume, 500 ml; respiratory rate, 12 breaths/min; and peak airway pressure, 28 cm H2O. He was unresponsive with bilateral constricted pupils; blood pressure was 95/50, and pulse was 110 beats/min. Air entry was adequate bilaterally with mild expiratory rhonchi. The extent of subcutaneous emphysema at the base of the neck was unchanged from the time of admission. Chest radiograph at this time showed mild diffuse parenchymal disease in the right lower lobe, left lingula, and left lower lobe. Pneumomediastinum and pneumoperitoneum were also evident. During the next 6 h, the patient’s level of consciousness gradually improved, and by the following morning, he was alert, oriented, and had no residual neurologic defect. A repeat FOB revealed swollen erythematous tracheobronchial mucosa but no wheat grains. The patient was then extubated. Tachypnea gradually resolved over the next 72 h, and his gas exchange improved progressively. He was observed in the SICU for 48 h, then transferred to the thoracic surgical ward. He continued to intermittently cough up wheat grains for the next 7 days. He was discharged from hospital 10 days after admission; at that time, pulmonary function tests showed a moderate restrictive pattern: FEV1/FVC = 1.96/2.371 (predicted = 3.40/3.96) with no response to bronchodilators and a borderline abnormal diffusing capacity for carbon monoxide (DCO) 79% predicted. At a follow-up visit in the clinic 9 months after discharge, the patient had no residual neurologic or respiratory signs or symptoms, and pulmonary function tests had returned to normal: FEV1/FVC = 3.57/4.29, DCO= 102% predicted.
References
Potkin RT, Swenson ER: Resuscitation from severe acute hypercapnia. Determinants of tolerance and survival. Chest 1992; 102:1742-5.
Giesecke AH, Gerbershagen HU, Dortman C, Lee D: Comparison of the ventilating and injection bronchoscopes. Anesthesiology 1978; 38:279-303.
Nunn JF: Nunn’s Applied Respiratory Physiology, Fourth Edition. Oxford: Butterworth, Heinemann, 1993, pp 19.
McIntyre RC Jr, Haenel JB, Read RR, Burch JM, Moore EE: Cardiopulmonary effects of permissive hypercapnia in the management of adult respiratory distress syndrome. J Trauma 1994; 37:433-8.
Tuxen DV: Permissive hypercapnic ventilation. Am J Respir Crit Care Med 1994; 150:870-4.
Nunn JF: Nunn’s Applied Respiratory, Physiology, Fourth Edition. Oxford: Butterworth, Heinemann, 1993, pp 519-28.
Litt L, Gonzalez-Mendez R, Severinghaus JW, Hamilton WK, Shulesko J, Murphy-Boesch J, James TL: Cerebral intracellular change during supercarbia: An in vivo sup 31 P nuclear magnetic resonance study in rats. J Cereb Blood Flow Metab 1985; 5:537-44.