Removing a solid-cystic intraabdominal desmoid tumor at a gastro-pancreatic lesion
Background
Desmoid tumors (also called aggressive fibromatosis, deep musculoaponeurotic fibromatosis) are locally aggressive tumors with no potential for metastasis or dedifferentiation. Radiologically, they are usually non-specific, slow-growing solid masses that are difficult to distinguish from other soft tissue tumors, but they rarely show cystic lesions. Sporadic desmoid tumors can occur at any site in the body, and anatomic locations are classified into 3 regions: trunk/extremity, abdominal wall, and intraabdominal. Complete surgical resection is the only curative option, but intraabdominal desmoid tumors are often unresectable, because they often infiltrate the mesentery. Here, we report a case of an intraabdominal desmoid tumor that occurred at a gastro-pancreatic lesion with cystic features, and presents the successful laparoscopic resection of the tumor.
Case presentation
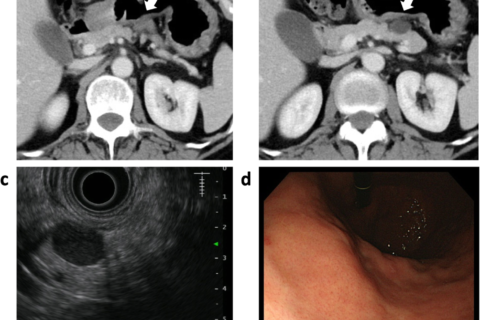
An intraabdominal cystic mass was incidentally detected between the stomach and the pancreas in a 52-year-old woman undergoing postoperative follow-up computed tomography (CT) for chondrosarcoma of a rib. Her family history was unremarkable. Blood counts and serum biochemistry results were within normal limits. An abdominal CT scan revealed a 20 × 18-mm well-defined cystic mass with a solid component (Fig. 1a,b). The tumor was adjacent to the stomach and the pancreas body, but there was no sign of invasive growth to the organs. MRI showed a cystic mass with high intensity on T2-weighted images and low intensity on T1-weighted images. The lesion showed no sign of diffusion restriction by diffusion-weighed MRI. The solid component of the cystic wall was gradually enhanced in the late phase. Subsequent endoscopic ultrasonography (EUS) showed an 18 × 13 mm round cystic mass, with a heterogeneous nodular lesion at the side of the serosa of the stomach (Fig. 1c). Esophago-gastric endoscopy showed no local lesion on the mucosal surface of the stomach (Fig. 1d). An intraabdominal tumor was suspected, but it was difficult to determine the specific diagnosis.
Laparoscopic resection via 5 trocars was performed with the patient in the supine position. After the gastro-colic ligament was cut, the omental bursa was opened widely. The tumor was confirmed at the superior border of tghe pancreas body, and adhered to the posterior wall of the stomach (Fig. 2a). With careful dissection, the left gastric artery and vein were isolated and preserved. The tumor was cut from the stomach by wedge resection of the gastric wall using a linear stapler (Fig. 2b). Then, we performed a spleen-preserving distal pancreatectomy. The pancreas body-tail was carefully mobilized and separated from the splenic artery and vein by dissecting the small vessels. The pancreas was dissected at the right side of the tumor using a linear stapler (Fig. 2c). We performed a complete laparoscopic excision, and the spleen and splenic vessels were completely preserved (Fig. 2d). The operative time was 279 min, and intraoperative blood loss was 15 mL. The postoperative course was uneventful, and the patient was discharged healthy.

Intraoperative findings. a The tumor was located in the gastro-pancreatic region and locally invaded the gastric wall and the pancreatic parenchyma. The arrow indicates the tumor. b Wedge resection of the stomach was performed using a linear stapler. c The pancreatic parenchyma was transected using a linear stapler. The arrow indicates the tumor. d The splenic artery (red tape) and splenic vein (blue tape) were preserved. The arrow indicates pancreatic stump
Macroscopic examination of the resected specimen revealed a capsulated cystic mass measuring approximately 20 mm with serous content. The whitish solid nodule containing the cystic lesion existed at the wall of the stomach (Fig. 3a). Microscopic examination demonstrated the distribution of spindle tumor cells, partly arranged in a fascicular fashion and accompanied by a collagenous stroma (Fig. 3b, c). Mitotic figures were rarely seen. The tumor cells were attached to the muscular layer of the gastric wall (Fig. 3b). A benign cystic lesion was also observed, but its origin was not determined. Immunohistochemically, the spindle cells were diffusely positive for β-catenin protein in the nucleus (Fig. 3d), but negative for theα-SMA, desmin, S-100, c-kit, and CD34 proteins. These findings were consistent with the diagnosis of intraabdominal desmoid tumor.

Macroscopic and microscopic findings. a The cut surface of the excised solid-cystic mass showing light tan glistening tissue. The arrow indicates the tumor. b A lower power view shows that the tumor cells (asterisk) originated from the gastric wall and attached to the pancreatic parenchyma without invasion (hematoxylin and eosin staining; original magnification × 40). c Histopathological findings show uniform cord-like proliferation of spindle-shaped cells with minimal atypia, and the intercellular spaces are filled with thick bundles of collagen fibers (hematoxylin and eosin staining; original magnification × 100). d Immunohistochemically, the spindle cells are diffusely and strongly positive for nuclear β-catenin protein. (× 100)
- 1.Reitamo JJ, Hayry P, Nykyri E, Saxen E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77(6):665–73.
- 2.Gurbuz AK, Giardiello FM, Petersen GM, Krush AJ, Offerhaus GJ, Booker SV, et al. Desmoid tumours in familial adenomatous polyposis. Gut. 1994;35(3):377–81.
- 3.Dinauer PA, Brixey CJ, Moncur JT, Fanburg-Smith JC, Murphey MD. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics. 2007;27(1):173–87.
- 4.Cholongitas E, Koulenti D, Panetsos G, Kafiri G, Tzirakis E, Thalasinou P, et al. Desmoid tumor presenting as intra-abdominal abscess. Dig Dis Sci. 2006;51(1):68–9.
- 5.Ko SF, Lin JW, Ng SH, Huang CC, Wan YL, Huang HY, et al. Spontaneous isolated mesenteric fibromatosis: sonographic and computed tomographic findings with pathologic correlation. Ultrasound Med Biol. 2006;32(8):1141–9.
- 6.Amiot A, Dokmak S, Sauvanet A, Vilgrain V, Bringuier PP, Scoazec JY, et al. Sporadic desmoid tumor. An exceptional cause of cystic pancreatic lesion. JOP. 2008;9(3):339–45.
- 7.Tan CH, Pua U, Liau KH, Lee HY. Mesenteric desmoid tumour masquerading as a fat-containing cystic mass. Br J Radiol. 2010;83(994):e200–3.
- 8.Xu B, Zhu LH, Wu JG, Wang XF, Matro E, Ni JJ. Pancreatic solid cystic desmoid tumor: case report and literature review. World J Gastroenterol. 2013;19(46):8793–8.
- 9.Mourra N, Ghorra C, Arrive L. An unusual solid and cystic pancreatic tumor in a 20-year-old woman. Desmoid Tumor. 2015;149(3):e5–6.
- 10.Patel HD, Desai NR, Som A, Shah SK, Thosani NC. Solid-cystic pancreatic tail Desmoid tumor with Beta-catenin positivity. ACG Case Rep J. 2017;4:e40.
- 11.Lee KC, Lee J, Kim BH, Kim KA, Park CM. Desmoid-type fibromatosis mimicking cystic retroperitoneal mass: case report and literature review. BMC Med Imaging. 2018;18(1):29.
- 12.Miyata K, Fukaya M, Nagino M. Gradually shrinking intra-abdominal desmoid tumor derived from the stomach in a young boy: a case report. Surg Case Rep. 2017;3(1):54.
- 13.Date K, Shima Y, Okabayashi T, Iwata J, Sumiyoshi T, Kozuki A. Desmoid tumor of the stomach. Endoscopy. 2015;47(Suppl 1):UCTN:E242–3.
- 14.Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17(1):158–67.
- 15.Malleo G, Damoli I, Marchegiani G, Esposito A, Marchese T, Salvia R, et al. Laparoscopic distal pancreatectomy: analysis of trends in surgical techniques, patient selection, and outcomes. Surg Endosc. 2015;29(7):1952–62.
- 16.Nakata K, Shikata S, Ohtsuka T, Ukai T, Miyasaka Y, Mori Y, et al. Minimally invasive preservation versus splenectomy during distal pancreatectomy: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2018;25(11):476–88.
Affiliations
- Department of Hepatobiliary-Pancreatic Surgery, National Hospital Organization Kyushu Cancer Center, 3-1-1 Notame, Minami-ku, Fukuoka, 811-1395, Japan
- Keishi Sugimachi
- , Tomohiro Iguchi
- & Yohei Mano
- Department of Gastroenterological Surgery, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
- Mitsuhiko Ohta
- , Masahiko Ikebe
- , Masaru Morita
- & Yasushi Toh
- Department of Hepato-Biliary-Pancreatology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
- Terumasa Hisano
- Department of Orthopedic Surgery, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
- Ryohei Yokoyama
- Department of Pathology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
- Kenichi Taguchi
Contributions
KS, TI, MO, YM and TH collected, analyzed and interpreted the patient data. RY and KT performed and interpreted the histological examination. KS, MI, MM and YT were responsible for study design, writing and scientific revision. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Keishi Sugimachi.