Foveal Eversion: A Possible Biomarker of Persistent Diabetic Macular Edema
 Emanuela Aragona, Luigi Capone, Rosangela Lattanzio, Piero Zollet, and Francesco Bandello
Emanuela Aragona, Luigi Capone, Rosangela Lattanzio, Piero Zollet, and Francesco BandelloAlessandro Arrigo
Department of Ophthalmology, Scientific Institute San Raffaele Hospital, University Vita-Salute, Milan, Italy
Emanuela Aragona
Department of Ophthalmology, Scientific Institute San Raffaele Hospital, University Vita-Salute, Milan, Italy
Luigi Capone
Department of Ophthalmology, Scientific Institute San Raffaele Hospital, University Vita-Salute, Milan, Italy
Rosangela Lattanzio
Department of Ophthalmology, Scientific Institute San Raffaele Hospital, University Vita-Salute, Milan, Italy
Piero Zollet
Department of Ophthalmology, Scientific Institute San Raffaele Hospital, University Vita-Salute, Milan, Italy
Francesco Bandello
Department of Ophthalmology, Scientific Institute San Raffaele Hospital, University Vita-Salute, Milan, Italy
 Corresponding author.
Corresponding author.Abstract
Introduction
We aimed to evaluate the impact of foveal eversion on treatment response and persistent diabetic macular edema (DME).
Methods
The study was designed as interventional and prospective. DME eyes were treated with ranibizumab and/or dexamethasone (DEX) implants, or with fluocinolone acetonide (FAc) implants. FAc-treated eyes were eventually retreated by additional ranibizumab injections. Main outcome measure was the relationship between foveal eversion and both clinical outcome and persistent DME.
Results
Sixty-eight DME eyes (68 patients) treated by anti-VEGF/DEX and 50 FAc-treated eyes (50 patients) were recruited. The follow-up was 16 ± 3 months. The anti-VEGF/DEX group and FAc-treated group were statistically matched for age, sex, DME duration and previous number of injections (p > 0.05). Both groups experienced statistically significant improvements of both BCVA and central macular thickness (p < 0.01) at the end of the follow-up. Persistent DME was shown by 46% of anti-VEGF/DEX eyes and 42% of FAc-treated eyes. Foveal eversion was found in 50% of anti-VEGF/DEX eyes and in 44% of FAc-treated eyes. Its presence was associated with worse anatomical and visual outcome and higher persistence of DME in both groups (p < 0.01) and with higher retreatment percentages in FAc-treated eyes (p < 0.01).
Conclusion
Foveal eversion is associated with worse clinical and morphological outcomes in DME.
Key Summary Points
| Diabetic macular edema (DME) is nowadays well managed by intravitreal anti-VEGF or corticosteroids treatments. |
| However, a considerable number of DME eyes, at least 30–40%, develop a vision-threatening condition called persistent DME. |
| In the present study, we investigated a specific structural optical coherence tomography (OCT) biomarker, namely foveal eversion, and assessed its relationship with treatment response and persistent DME. |
| Foveal eversion was significantly associated with worse anatomical and visual outcome and significantly higher prevalence of persistent DME. |
| In the perspective of even more personalized treatment strategies, foveal eversion may be considered a feasible structural OCT biomarker in DME. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13360130.
Introduction
Diabetic macular edema (DME) is a cause of visual acuity loss in diabetic retinopathy (DR) [1, 2]. Nowadays, DME is well managed by means of intravitreal anti-VEGF injections and corticosteroids [3], including the recently introduced fluocinolone acetonide (FAc) 0.19 mg intravitreal drug-delivery system (ILUVIEN®; Alimera Sciences, Inc., Alpharetta, GA, USA), which is able to guarantee a longer duration of the treatment [4–9]. Although the usage of intravitreal treatments radically changed the clinical course of DME, a considerable percentage of patients, at least 30–40%, showed persistent DME [10]. This condition leads to a chronicity of the clinical picture, with a progressive anatomical and functional impairment of the macula and thus a worse visual prognosis. Although previous studies tried to assess the relationship between imaging biomarkers and DME remission [11–14], fewer data are available regarding the role of imaging in predicting DME persistence. Although this feature is important in each clinical setting, it assumes a remarkable relevance in the setting of FAc implants, especially to plan additional treatments.
The main aim of the present study was to investigate a specific structural optical coherence tomography (OCT) biomarker, namely foveal eversion, defined as a complete convex profile of the central fovea, in DME patients who underwent anti-VEGF or corticosteroid intravitreal treatments to assess the possible relationship with treatment response. Furthermore, we assessed the relationship with persistent DME, intended as the still present DME through the 24-week visit [15].
Methods
The study was designed as a prospective, cohort study involving DME patients followed at the Department of Ophthalmology, San Raffaele Hospital, Milan, Italy. The study was approved by the Ethics Committee of San Raffaele Hospital, Milan, Italy, and was conducted in accordance with the Helsinki Declaration. We obtained signed informed consent from all the patients before inclusion into the study.
The inclusion criterion was the presence of a clinically relevant DME, treated for at least 6 months before our baseline visit. Exclusion criteria were: naïve DME, phakic eye, other media opacities, uncontrolled glaucoma, any ophthalmic surgery in the last 6 months before treatment, macular edema secondary to other causes than DME, and any ophthalmic or systemic disease potentially affecting the results of the study. The follow-up was at least 1 year for all the included eyes.
All the patients underwent complete ophthalmologic examination including best corrected visual acuity (BCVA) evaluated by standard ETDRS charts, anterior and posterior segment slit-lamp evaluation, and Goldmann applanation tonometry. We performed structural OCT examination (Spectralis HRA, Heidelberg Engineering; Heidelberg, Germany) at each follow-up, with radial, raster and dense scans with high numbers of frames (ART > 25) and enhanced depth imaging (EDI) to highlight deeper structures.
From structural OCT images, we extracted the following parameters: central macular thickness (CMT), inner retinal thickness (IRT), outer retinal thickness (ORT), disorganization of the inner retinal layers (DRIL), epiretinal membrane (ERM), ellipsoid zone (EZ) and external limiting membrane (ELM) status, retinal hyperreflective foci (HF), subretinal fluid (SRF) and the presence of foveal eversion. This latter was defined as a complete convex profile of the central fovea.
We separately analyzed eyes with or without foveal eversion. We treated DME with anti-VEGF (ranibizumab 0.5 mg) and/or dexamethasone (DEX) implant (Ozurdex®; Allergan inc.), or with FAc implant. Anti-VEGF injections and DEX implants were administered accordingly to a pro-re-nata regimen. In particular, DME eyes could be equally treated with one of these drugs, according to the ophthalmologists’ discretion. The criterion to switch the treatment was the OCT-based evidence of poor response to the first molecule (arbitrarily considering DME reduction < 25%). FAc implant was administered according to the Italian guidelines, consisting of the following characteristics: refractory DME, previously treated with at least one DEX implant and without the evidence of “cortico-responder”-related phenomena, in mandatory pseudophakic eyes. FAc-treated eyes could eventually be retreated with anti-VEGF, if detecting structural OCT evidence of poor response.
With respect to the eyes treated by anti-VEGF/DEX, the choice to include only pseudophakic eyes was also made to make all the DME eyes comparable, considering the mandatory criterion of administration of the FAc implant. Furthermore, the exclusion of naïve DME eyes was chosen to further guarantee a reliable statistical comparison between the anti-VEGF/DEX and FAc implant groups.
After the categorization of our cohort in DME eyes with or without foveal eversion, we also performed further analyses separately considering patients treated by anti-VEGF and/or DEX implant from patients who underwent FAc implant. We decided to consider FAc-treated eyes as a separate subgroup in consideration of the fixed and stable concentration of the drug released each month, with respect to an anti-VEGF and/or DEX implant, both of which are known to be characterized by a less stable intravitreal concentration of the drugs.
The main outcome measure was the assessment of the relationship between foveal eversion and both the final outcome and the presence of persistent DME, intended as the still present DME through the 24-week visit [15]. We considered the parameters measured at baseline and at the last follow-up. Two independent blinded graders performed all the measures (AA, EA). Interclass correlation coefficient (ICC) was measured to assess the agreement between the two graders through a two-way random-effects model. We considered the following parameters as fixed factors in the analysis: age, gender, systemic hypertension, type and duration of diabetes mellitus (DM), glycate hemoglobin (HbA1c), DR type (NPDR/PDR), duration of DME, previous history of vitrectomy, previous panretinal photocoagulation (PRP), previous nature and number of treatments (anti-VEGF, intravitreal corticosteroids), baseline features (BCVA and CMT), retinal and/or choroidal HF > 15, ERM, DRIL, ELM/EZ status (normal/interrupted/absent) and SRF. In the FAc-treated group, we also considered the eventual presence of additional anti-VEGF treatment (yes/no) and their number, administered in accordance to the ophthalmologists’ discretion.
All the statistical analyses were performed using the SPSS software package (SPSS, Chicago, IL, USA). Continuous variables were statistically evaluated by means of two-tailed t test. One-way ANOVA analysis assessed the differences between eyes disclosing foveal eversion and eyes without foveal eversion for both treatment groups. Because of multiple testing, Bonferroni correction was applied to assess for multiple comparisons. Tau-Kendall correlation analysis was adopted to assess the relationship among the included parameters. Statistical significance was set at p < 0.05.
Results
We collected data from 68 DME eyes of 68 patients (42 males; mean age 66 ± 7 years) treated with anti-VEGF injections and DEX implants and from 50 eyes of 50 patients (29 males; mean age 68 ± 9 years) treated with FAc implants. The two groups were age- and sex-matched (p > 0.05). The mean follow-up was 16 ± 3 months for both groups. The mean number of previous intravitreal injections was 11 ± 2 for the anti-VEGF/DEX group and 10 ± 3 for the FAc-treated group (p > 0.05). The mean DME diagnosis resulted 2 ± 1 years for both groups (p > 0.05). The anti-VEGF/DEX group was treated with 9 ± 2 injections over the entire follow-up. Only 10 out of 68 eyes (15%) were treated by only anti-VEGF or DEX, without switching. Clinical data are extensively reported in Table Table11.
Table 1
Clinical data in anti-VEGF/DEX- and FAc-treated DME eyes
| Clinical data | ||||
|---|---|---|---|---|
| Parameter | Anti-VEGF/DEX group | FAc implant group | ||
| Mean ± STD | p value | Mean ± STD | p value | |
| Number of eyes | 68 | 50 | ||
| Age | 66 ± 7 | 68 ± 9 | ||
| Gender (M/F) | 42/36 | > 0.05 | 29/21 | > 0.05 |
| DM type (I/II) % | 41%/59% | < 0.01 | 38%/62% | < 0.01 |
| DR type (NPDR/PDR) % | 51%/49% | > 0.05 | 46%/54% | > 0.05 |
| Hb1Ac value % | 7.0 ± 0.7 | 7.2 ± 0.9 | ||
| Arterial hypertension % | 60% | 54% | ||
| Previous focal/grid laser % | 35% | 32% | ||
| Previous anti-VEGF % | 90% | 72% | ||
| Previous DEX % | 78% | 90% | ||
| Previous PRP % | 55% | 48% | ||
| Previous vitrectomy % | 22% | 16% | ||
| LogMAR BCVA baseline | 0.55 ± 0.38 | 0.02 | 0.51 ± 0.35 | 0.02 |
| LogMAR BCVA last | 0.43 ± 0.33 | 0.44 ± 0.31 | ||
| CMT baseline | 555 ± 132 | < 0.01 | 476 ± 125 | < 0.01 |
| CMT last | 315 ± 121 | 327 ± 107 | ||
| DME duration > 3 months % | 89% | 86% | ||
| DRIL baseline % | 18% | 0.02 | 10% | 0.02 |
| DRIL last % | 30% | 22% | ||
| EZ/ELM status baseline % (preserved/disrupted/absent) | 29%/71%/0% | > 0.05 | 28%/72%/0% | > 0.05 |
| EZ/ELM status last % (preserved/disrupted/absent) | 32%/65%/3% | 32%/66%/2% | ||
| SRF baseline % | 29% | < 0.01 | 20% | < 0.01 |
| SRF last % | 0% | 0% | ||
| HF > 15 baseline % | 88% | > 0.05 | 90% | > 0.05 |
| HF > 15 last % | 81% | 84% | ||
| ERM baseline % | 26% | 22% | ||
| Foveal eversion baseline % | 50% | > 0.05 | 44% | > 0.05 |
| Foveal eversion last % | 54% | 48% | ||
| Additional anti-VEGF treatment % | N/A | 28% | ||
| Persistent DME last | 46% | 42% | ||
DR diabetic retinopathy, DME diabetic macular edema, PRP panretinal photocoagulation, DEX dexamethasone, BCVA best corrected visual acuity, CMT central macular thickness, CT choroidal thickness
The anti-VEGF/DEX group and FAc-treated group did not significantly differ in terms of clinical and imaging parameters (Table (Table1)1) (all p > 0.05). Both groups showed a significant improvement of LogMAR BCVA and CMT at the end of the follow-up compared to baseline values (Table (Table11).
Persistent DME was detected in 46% of anti-VEGF/DEX eyes and in 42% of FAc-treated eyes (p > 0.05).
We found foveal eversion in 34 out of 68 eyes (50%) of anti-VEGF/DEX group and in 22 out of 50 eyes (44%) of the FAc-treated group (Fig. 1).
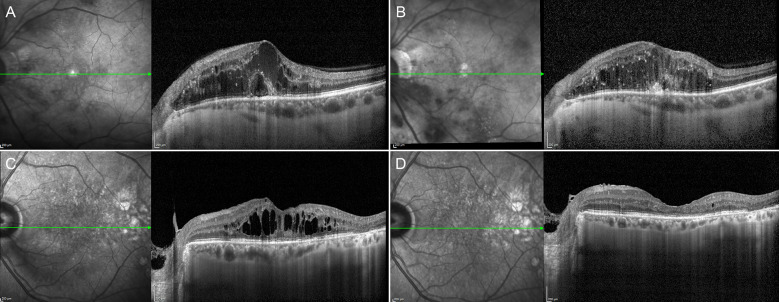
Representative case of foveal eversion. The presence of foveal eversion (a) is associated with the incomplete recovery of the macular profile and persistent DME after treatment (b). On the other side, the presence of DME without an everted foveal profile (c) is related to the complete absorption of the edema secondary to intravitreal treatments (d)
DME eyes with and without foveal eversion started with similar baseline BCVA values (p > 0.05) (Table (Table2).2). A statistically significant BCVA improvement was registered only in eyes without foveal eversion, for both the anti-VEGF/DEX and FAc-treated groups. On the other hand, the eyes disclosing foveal eversion were characterized by stable BCVA, if considering baseline and last follow-up values (p > 0.05).
Table 2
Foveal eversion sub-analysis in anti-VEGF/DEX and FAc-treated DME eyes
| Foveal eversion analysis | ||||||
|---|---|---|---|---|---|---|
| Parameter | Anti-VEGF/DEX group | FAc implant group | ||||
| No foveal eversion | Foveal eversion | p value | No foveal eversion | Foveal eversion | p value | |
| Number of patients | 34 | 34 | > 0.05 | 28 | 22 | > 0.05 |
| Age | 66 ± 8 | 65 ± 9 | > 0.05 | 67 ± 8 | 70 ± 9 | > 0.05 |
| Gender (M/F) | 23/11 | 19/15 | > 0.05 | 14/14 | 15/7 | > 0.05 |
| DM type (I/II) % | 53%/47% | 29%/71% | 0.01 | 54%/46% | 23%/77% | 0.02 |
| DR type (NPDR/PDR) % | 32%/68% | 65%/35% | 0.01 | 36%/64% | 59%/41% | 0.02 |
| Hb1Ac value % | 6.9 ± 0.7 | 7.2 ± 0.8 | > 0.05 | 7.2 ± 0.9 | 7.2 ± 0.9 | > 0.05 |
| Arterial hypertension % | 24% | 97% | < 0.01 | 39% | 77% | 0.02 |
| Previous focal/grid laser % | 29% | 41% | > 0.05 | 25% | 41% | > 0.05 |
| Previous anti-VEGF % | 88% | 91% | > 0.05 | 79% | 64% | > 0.05 |
| Previous DEX % | 74% | 82% | > 0.05 | 86% | 91% | > 0.05 |
| Previous PRP % | 62% | 47% | > 0.05 | 54% | 45% | > 0.05 |
| Previous vitrectomy % | 29% | 15% | > 0.05 | 25% | 5% | > 0.05 |
| LogMAR BCVA baseline | 0.51 ± 0.35 | 0.61 ± 0.36 | > 0.05 | 0.49 ± 0.34 | 0.52 ± 0.37 | > 0.05 |
| LogMAR BCVA last | 0.39 ± 0.25 | 0.50 ± 0.36 | 0.02 | 0.40 ± 0.28 | 0.49 ± 0.34 | 0.02 |
| CMT baseline | 524 ± 101 | 575 ± 112 | > 0.05 | 484 ± 97 | 542 ± 128 | > 0.05 |
| CMT last | 268 ± 51 | 401 ± 125 | < 0.01 | 277 ± 59 | 390 ± 121 | < 0.01 |
| DME duration > 3 months % | 82% | 97% | > 0.05 | 82% | 91% | > 0.05 |
| DRIL baseline % | 12% | 24% | 0.02 | 7% | 14% | > 0.05 |
| DRIL last % | 21% | 38% | 0.03 | 14% | 32% | 0.03 |
| EZ/ELM status baseline % (preserved/disrupted/absent) | 35%/65/0% | 24%/76%/0% | 0.02 | 36%/64/0% | 18%/82%/0% | 0.02 |
| EZ/ELM status last % (preserved/disrupted/absent) | 29%/71%/0% | 35%/59%/6% | 0.02 | 43%/57%/0% | 18%/77%/5% | 0.02 |
| SRF baseline % | 35% | 24% | > 0.05 | 25% | 14% | > 0.05 |
| SRF last % | 0% | 0% | > 0.05 | 0% | 0% | > 0.05 |
| HF > 15 baseline % | 76% | 100% | 0.02 | 86% | 93% | > 0.05 |
| HF > 15 last % | 62% | 100% | 0.02 | 77% | 82% | > 0.05 |
| ERM baseline % | 21% | 32% | > 0.05 | 14% | 32% | > 0.05 |
| Foveal eversion baseline % | 0% | 100% | < 0.01 | 0% | 100% | < 0.01 |
| Foveal eversion last % | 12% | 100% | < 0.01 | 7% | 100% | < 0.01 |
| Additional anti-VEGF treatment % | N/A | N/A | N/A | 14% | 45% | < 0.01 |
| Persistent DME last | 12% | 79% | < 0.01 | 21% | 68% | < 0.01 |
BCVA best corrected visual acuity, CMT central macular thickness, DRIL disorganization of inner retinal layers, IRT inner retinal thickness, ORT outer retinal thickness, SRF subretinal fluid, CT choroidal thickness, HF hyperreflective foci, ELM external limiting membrane, EZ ellipsoid zone
CMT was similar at baseline in eyes with or without foveal eversion (p > 0.05), considering both groups of DME eyes; on the contrary, it turned out to be significantly higher in the foveal eversion eyes at the end of the follow-up in both the anti-VEGF/DEX group and FAc-treated group (p < 0.01).
Interestingly, the presence of persistent DME at the end of the follow-up was significantly higher in foveal eversion eyes (76% of eyes in the anti-VEGF/DEX group and 68% of eyes in the FAc-treated group) compared to eyes without foveal eversion (~ 20% in both groups) (p < 0.01).
Independently from the presence of foveal eversion, the prevalence of ERM and SRF was similar between groups, with SRF being completely recovered at the end of the follow-up in 100% of cases.
The percentages of eyes with DME duration > 3 months was homogeneously distributed in eyes with or without foveal eversion for both the anti-VEGF/DEX group and in FAc-treated group.
Furthermore, the presence of foveal eversion was also significantly associated with worse EZ/ELM status, a higher percentage of DRIL and higher HF (Table (Table2).2). Specifically, DME eyes with foveal eversion had a significantly higher percentage of EZ/ELM disruption at baseline than eyes without foveal eversion; moreover, EZ/ELM absence was detected only in eyes with foveal eversion at the end of the follow-up in both the anti-VEGF/DEX and FAc-treated groups. Previous vitrectomy had no influence in terms of number of injections, presence of foveal eversion and outcome (all p > 0.05). All the other evaluated parameters were similar between the two groups (p > 0.05).
Remarkably, in the anti-VEGF/DEX group, DME eyes disclosing foveal eversion underwent significantly more intravitreal injections than DME eyes without foveal eversion (10 ± 2 vs. 8 ± 2; p = 0.01).
With respect to FAc-treated eyes, the mean number of additional anti-VEGF injections was 2 ± 1, considering the overall sample of 50 eyes. The post-hoc analysis revealed that eyes with foveal eversion underwent significantly more additional anti-VEGF treatments than eyes without foveal eversion (45% vs. 14% of eyes; p < 0.01) (Table (Table22).
The inter-grader agreement regarding the detection of foveal eversion was ICC 0.97 (p < 0.01); moreover, the overall ICC regarding the other parameters analyzed was 0.93 (range 0.90–0.96; p < 0.01).
The correlation analysis (Table (Table3)3) confirmed the relationship of foveal eversion with higher CMT values and higher frequency of persistent DME at the end of the follow-up. Moreover, our data showed that patients with type 2 DM and arterial hypertension are more subject to the foveal eversion phenomenon. The choice to re-treat FAc-treated DME eyes with anti-VEGF was strictly related to the amount of DME. The number of injections in the anti-VEGF/DEX group correlated with the persistence of DME. Moreover, looking at the FAc-treated group, eyes with proliferative DR, previously treated by PRP, showed negative correlation with anti-VEGF retreatment. EZ/ELM status strictly depended on the presence of DME duration > 3 months (Table (Table33).
Table 3
Correlation analysis
| Correlation analysis | |||||
|---|---|---|---|---|---|
| Parameter | CMT last | EZ/ELM status | Persistent DME | DM type | Arterial hypertension |
| Foveal eversion | |||||
| Tau-Kendall coeff. | 0.543 | 0.444 | 0.697 | 0.383 | 0.553 |
| p value | < 0.001 | 0.01 | < 0.001 | 0.02 | 0.01 |
| Parameter | PRP | Focal/grid laser | Vitrectomy | Re-treat |
|---|---|---|---|---|
| DR type | ||||
| Tau-Kendall coeff. | 0.825 | − 0.405 | 0.442 | − 0.461 |
| p value | < 0.001 | < 0.001 | 0.01 | < 0.001 |
| Parameter | CMT last | PRP |
|---|---|---|
| Re-treat | ||
| Tau-Kendall coeff. | 0.440 | − 0.409 |
| p value | < 0.001 | < 0.001 |
| Parameter | Persistent DME | CMT last |
|---|---|---|
| No. of injections | ||
| Tau-Kendall coeff. | 0.514 | 0.498 |
| p value | < 0.001 | < 0.001 |
| Parameter | DME duration > 3 months |
|---|---|
| EZ/ELM status | |
| Tau-Kendall coeff. | 0.615 |
| p value | < 0.001 |
| Parameter | Focal/grid laser |
|---|---|
| PRP | |
| Tau-Kendall coeff. | − 0.443 |
| p value | < 0.001 |
DR diabetic retinopathy, DM diabetes mellitus, DME diabetic macular edema, CMT central macular thickness, ELM external limiting membrane, EZ ellipsoid zone, PRP panretinal photocoagulation
Discussion
In the present study, we evaluated the impact of foveal eversion, detected on structural OCT, on the response to anti-VEGF/DEX treatment or FAc implant over at least 1-year follow-up and on the persistence of DME. Our data revealed a significant association between the presence of foveal eversion and both visual and anatomical outcomes. In particular, we found significant BCVA and CMT improvements only in DME eyes without foveal eversion. Nevertheless, the intravitreal treatments showed a good efficacy in maintaining stable BCVA values also in eyes disclosing foveal eversion. An interesting result was the completely different prevalence of persistent DME in eyes with or without foveal eversion (76% vs. 20% in the anti-VEGF/DEX group and 68% vs. 21% in FAc-treated group after at least 1-year follow-up).
It should be considered that, to date, a definite agreement about the prevalence of persistent DME is still lacking. We could consider a prevalence of at least 40% of cases reliable [15], although high data variability is reported in the literature. From this point of view, our findings matched with previous reports, disclosing persistent DME in 46% of anti-VEGF/DEX eyes and in 42% of FAc-treated eyes. Remarkably, our analysis allowed to categorize our DME cohort in eyes with an extremely high probability to show persistent DME from eyes with just 20% of cases in an extremely feasible way, namely the structural OCT detection of foveal eversion. This finding suggests a useful role of the foveal eversion biomarker in predicting persistent DME and in planning personalized treatment strategies.
The eversion of the foveal profile, intended as a complete convex profile of the central fovea, was a poorly investigated structural OCT biomarker, detectable in different macular diseases. It was suggested to be related with worse prognosis in retinal vein occlusion [16]. Moreover, its absence has already been suggested as a possible exclusion selection criterion for FAc implants to achieve good visual outcome [17].
The interesting aspect of foveal eversion is the extremely easy practicability of assessing its presence in common clinical settings. Indeed, foveal eversion can be intended as a dichotomic biomarker (present/absent), without the need of more complex quantitative methods for its evaluation.
The anatomical explanation of foveal eversion is quite difficult. A previous DME study described its relationship with precise cytokine profiles as different from other forms of DME [18]. This is in accordance with the extremely complex and heterogeneous pathogenesis of DME, including completely different pathogenic sources of macular edema [19, 20].
On the other hand, considering the strong involvement of intraretinal glial cells, in particular of Muller cells, in DME [21–23], the onset of foveal eversion might be related to a stronger impairment of the Muller cells. Indeed, these cells are known to cover several fundamental metabolic roles within the retina, including the release of growth factors and other metabolic and inflammatory mediators [24]. The highest concentration of Muller cells is localized at the level of the fovea, with a 1:1 ratio between Muller cells and photoreceptors [25]. For this reason, a major impairment of this specific cytotype might be responsible for a remarkable loss of foveal structural and functional integrity, leading to the onset of foveal eversion. Moreover, the consequent loss of macular homeostasis might also justify the higher prevalence of persistent DME in eyes with foveal eversion. From this point of view, the presence of higher biomarkers of retinal impairment (DRIL and HF) in foveal eversion eyes suggests a wider damage of the retinal cytotypes, with the consequent interpretation of foveal eversion as the final effect of the combined activity of greater inflammation and greater retinal structural loss.
However, we know that all these speculations need further studies, including histologic validations to better investigate the pathogenesis of foveal eversion and its relationship with treatment response and persistent DME.
We are aware that our study labors under several limitations, mainly related to the relatively low number of patients and the limited follow-up. For this reason, ours should be considered just a first investigation stimulating future larger studies dedicated to the evaluation of foveal eversion in DME. Furthermore, we did not include naïve DME. This choice was taken to make the two considered groups statistically comparable. Indeed, in accordance with the Italian guidelines, DME patients can undergo FAc implants only as second-line treatment. Future studies are warranted to assess foveal eversion features and impact also in naïve DME eyes. Lastly, the percentage of DME eyes treated with only anti-VEGF or DEX implant was too low (15% of the entire cohort) to make a reliable statistical analysis dedicated on the impact of foveal eversion in DME eyes treated without switching. For all these reasons, our findings will benefit from further validations provided by larger prospective investigations.
Conclusions
In conclusion, our study gained new insights into the role of foveal eversion for the assessment of DME response to anti-VEGF/DEX and FAc implant treatments as well as for the prediction of persistent DME. In the perspective of even more optimized and personalized therapeutic strategies, foveal eversion might represent an easily evaluable biomarker in DME eyes. Further studies are warranted to assess the role of foveal eversion in the pathogenesis of DME.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Francesco Bandello: consultant for Alcon (Fort Worth, TX, USA), Alimera Sciences (Alpharetta, GA, USA), Allergan Inc. (Irvine, CA, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, NY, USA), Genentech (San Francisco, CA, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium) and Zeiss (Dublin, CA, USA). Alessandro Arrigo, Emanuela Aragona, Luigi Capone, Rosangela Lattanzio and Piero Zollet have nothing to disclose.
Compliance with Ethics Guidelines
The study was designed as prospective, cohort study involving DME patients followed at the Department of Ophthalmology, San Raffaele Hospital, Milan, Italy. The study was approved by the Ethics Committee of San Raffaele Hospital, Milan, Italy, and was conducted in accordance with Helsinki Declaration. We obtained signed informed consent from all the patients before inclusion into the study.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.