Tuberculous Cutaneous Ulcers Associated with Miliary Tuberculosis in an Elderly Woman
Laurence Toutous-Trellu
cDepartment of Dermatology and Venerology, University Hospitals of Geneva, Geneva, Switzerland
Isabelle Charlet
aDepartment of Geriatric, University Hospitals of Geneva, Geneva, Switzerland
Bernard Hirschel
bDepartment of Infectious Diseases, University Hospitals of Geneva, Geneva, Switzerland
C. Prins
cDepartment of Dermatology and Venerology, University Hospitals of Geneva, Geneva, Switzerland
I. Masouyé
cDepartment of Dermatology and Venerology, University Hospitals of Geneva, Geneva, Switzerland
Ulrich M. Vischer
aDepartment of Geriatric, University Hospitals of Geneva, Geneva, Switzerland
Jean-Paul Janssens
dDepartment of Pulmonary Diseases Unit, University Hospitals of Geneva, Geneva, Switzerland
Abstract
Skin localizations in disseminated tuberculosis may present a clinical resistant evolution. An 81-year-old woman, treated by long-term steroids and methotrexate for rheumatoid polyarthritis, developed a disseminated tuberculosis in chest, bones and skin. While pulmonary symptoms quickly improved under conventional tuberculostatic drugs, skin ulcers showed positive cultures for 5 months and healed after 12 months of treatment.
Case Report
An 81-year-old woman was admitted to hospital in June 2005 because of a persistent cough with fever in spite of a course of oral antibiotics (co-amoxiclav) prescribed by her general practitioner. The symptoms had developed progressively over 1 month. She was on a long-term treatment of prednisone 5 mg/day and methotrexate 4 mg/week for rheumatoid arthritis diagnosed in 1990.
On admission, the patient complained of asthenia, anorexia, weight loss (4 kg) and persistent fever (ca. 39°C). She was febrile (38°C) and in poor general condition. Crackles were audible over both pulmonary bases. She had bilateral deformities of her fingers but no signs of active inflammatory arthritis. There was, however, a swelling of the right hand with painless necrotic ulcerations of the palm and middle finger surrounded by erythema (fig. 1). A large painless necrotic ulcer was also present below the popliteal area of her right knee (fig. 2 a). Bilateral symmetric and violaceous edema of the lower limbs was also noted. Two pustules were present on the left leg.
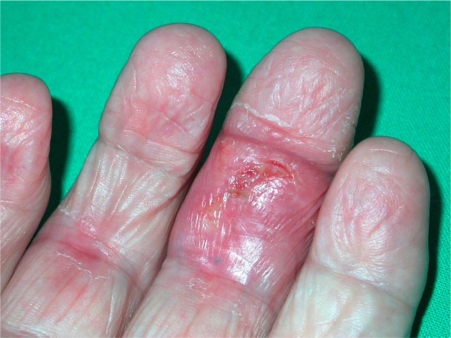
Painless tuberculous ulcer of the third finger.

a Violaceous edema of the leg and popliteal necrosis. b 3 months later, evolution in a large necrotic ulcer.
Laboratory tests showed a mixed inflammatory and folate-deficiency anemia (hemoglobin 88 g/l, normal: 120–160 g/l; folate 2.3 nmol/l, normal: 6–35.5 nmol/l), a normal white blood count (10.6 ×109/l, normal: 4–11 ×109/l), a high C reactive protein (173 mg/l, normal: 0–9 mg/l), and a low serum albumin (17 g/l, normal: 30–40 g/l); liver tests and kidney function were normal.
Chest X ray and CT scan revealed a micro-nodular infiltrate, without any abnormalities at the abdominal level. MRI showed multiple small lacunae in the right second and fifth fingers, and at the distal end of the right femur. These lesions were considered compatible with either a tuberculous bone involvement or rheumatoid arthritis.
Tuberculin test (2 U of RT23 Tuberculin, Statens Serum Institute, Copenhagen, DK) was negative at 48 h. Result of T-SPOT.TB γ-interferon assay was indeterminate.
Acid fast bacilli (AFB) were seen in bronchoalveolar lavage, in smears from the popliteal ulcer and in the skin biopsy from the third finger of the right hand. PCR and cultures were positive for Mycobacterium tuberculosis, sensitive to all first-line tuberculostatic drugs. Histopathological examination of the skin biopsy from the right hand showed a granulomatous inflammatory infiltrate with lymphocytes, giant cells, macrophages and central necrosis (fig. 3 a). The Ziehl Nielson stain showed numerous AFB (fig. 3 b).

a Skin biopsy of the third finger (fig. 1) showing the granulomatous infiltrate and necrosis (hematoxylin and eosin). b Numerous AFB are present in the finger biopsy (Ziehl Nielson; original magnification ×630).
The patient was started on isoniazid 300 mg/day, ethambutol 800 mg/day, rifampicin 450 mg/day and pyrazinamide 1,250 mg/day. After an initial remission of pulmonary symptoms, a paradoxical reaction (unexplained and persistent fever) occurred after 6 weeks of treatment, requiring an increase of systemic steroids to 20 mg/day for 3 weeks. The digital and palmar ulcerations healed completely in 2 months. After 3 months, there was a remarkable improvement in the patient’s general condition. However, during the same period, the cutaneous involvement of the lower limbs progressed: a large and deep necrotic area appeared over the right calf, extending from the right popliteal ulcer (fig. 2 b), and a smaller but deep ulceration appeared over the left popliteal area. Skin swabs showed persistent AFB, with cultures remaining positive after 5 months of treatment. Two subsequent antibiograms did not disclose any new resistance to tuberculostatic drugs.
Because of the lack of clinical response of the lower limb ulcerations to conventional tuberculostatic treatment after 5 months, the patient was re-admitted to hospital. Moxifloxacin 400 mg daily was added to a 4-week intravenous treatment including rifampicin, isoniazid and ethambutol. Moreover, topical amikacin was applied on the ulcers for 8 weeks with good tolerance. The skin ulcers slowly improved and healed at the 12th month. AFB were persistently documented on direct examination skin swabs until the complete epidermization of the wound; however, skin cultures remained negative. Oral rifampicin, isoniazid and moxifloxacin were discontinued in June 2006, 1 year after start. One month later, the patient developed an acute pneumonia and died. Autopsy revealed necrotic granulomas and AFB in the lungs.
Discussion
In developed countries, for the indigenous population, tuberculosis (TB) is essentially a disease of the old and very old [1]. According to official statistics (available from the Swiss Federal office of public health, http://www.bag.admin.ch) in Switzerland, the incidence of TB in subjects aged 70–79 years is 14.5/105 inhabitants, 2.5 times higher than in the general population. The incidence is even 4.8 times higher for those aged 80 and above (27.6/105 inhabitants). Pulmonary involvement is the most frequent clinical presentation. Less than 15% of elderly patients present with extra-thoracic involvement [1]. A miliary pattern is reported in 4–7% of elderly subjects with TB, i.e. more frequently than in younger subjects [2, 3]. Cutaneous tuberculosis is unusual in industrialized countries, most reports coming from developing countries [4, 5, 6].
Skin lesions in our patient were most probably consecutive to hematogenous dissemination of mycobacteria from a pulmonary focus, creating metastatic abscesses. Such abscesses are known to produce ulcers or fistulas [2, 7]. In our patient, drug-induced immunosuppression and skin atrophy may also have contributed to the extensive septic emboli in small arteries, necrosis and ulcers. Cutaneous lesions usually respond favorably to antituberculous therapy; however, they sometimes require surgical management [8, 9]. Slow responders to treatment have been reported, with lesions persisting occasionally ≥1 year. The high density of bacilli in the skin lesions of this case was illustrated over the 1-year follow-up period, with persistent AFB in the skin ulcers, and negative results of cultures only after 5 months on treatment. Surprisingly, improvement of thoracic and bone involvement was much quicker than that of the skin lesions. Because of the slow resolution of the skin lesions after 5 months of treatment, we hypothesized that the local biodisponibility of tuberculostatic drugs was insufficient. However, we could not clearly demonstrate that reinforced tuberculosis therapy accelerated skin healing. Since the recommended duration of therapy in cutaneous tuberculosis resulting from hematogenous spread is 10–12 months [10], healing might have occurred even on the initial oral therapy. The use of topical antibiotherapy in cutaneous tuberculous ulcers has not been validated to date. Wound decontamination and scarring may have been delayed by prednisone therapy, which could not be lowered because of the active rheumatoid arthritis.
Moreover, this case shows that TB can not be excluded in aging people by the γ-interferon assay, and indeterminate results have been associated with old age, sensitivity reaching up to 80% in the elderly [11, 12].
The dramatic outcome illustrates the difficulty to evaluate the duration of tuberculosis treatment in aged immunosuppressed patients with such exceptional presentation.
Conclusion
Although cutaneous tuberculosis is quite unusual, tuberculosis deserves to be considered in patients with unexplained skin lesions or ulcers, even in elderly non-migrant patients. Ulcer healing is slow and may trail behind recovery in lungs and bone. Drug-induced immunosuppression can favor TB dissemination and delay the microbiological and clinical responses. However, prolonged treatment with multiple antibiotics and local wound care can slowly achieve healing of ulcers.
References
Content retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2895205/.